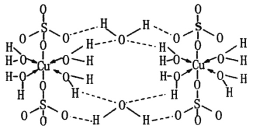
��Ŀ����
����Ŀ����ͼ��ʾ 25��ʱ��ϡ�� HClO��CH3COOH �������ϡ��Һʱ����Һ pH ���ˮ���ı仯���������˵������ȷ����
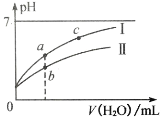
A.��ͼ��֪��Ϊ CH3COOH����Ϊ HClO
B.ͼ�� a ������Һ��Ũ�ȴ��� b ������Һ��Ũ��
C.ͼ�� a��c ���㴦����Һ��![]() ���(HR ���� CH3COOH �� HClO)
���(HR ���� CH3COOH �� HClO)
D.��ͬŨ�� CH3COONa �� NaClO �Ļ��Һ�У�������Ũ�ȵĴ�С��c(Na��)��c(CH3COO��)��c(ClO��)��c(OH��)��c(H��)
���𰸡�B
��������
A���������ԣ�CH3COOH��HClO������������ˮʱ��CH3COOH��pH�仯�������ͼ������ΪCH3COOH����ΪHClO��Aѡ����ȷ��
B����A��֪��CH3COOH�ĵ���̶ȴ���HClO��������ϡ����ͬ����ʱ������a��b����ʱ��CH3COOH��Ũ��С��HClO��Bѡ�����
C������������CH3COOH��CH3COOH�д��ڵ���ƽ��CH3COOH![]() CH3COO-+H+�������ƽ�ⳣ��
CH3COO-+H+�������ƽ�ⳣ��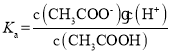 ����
����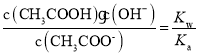 ��a��c�����¶���ͬ������Kw��Ka����ȣ���
��a��c�����¶���ͬ������Kw��Ka����ȣ���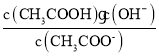 ��ȣ�Cѡ����ȷ��
��ȣ�Cѡ����ȷ��
D����ͬŨ��CH3COONa��NaClO�Ļ��Һ�У�����CH3COO-��ClO-����ˮ���Լ��ԣ���ˮ��̶�CH3COO-��ClO-�������Һ������Ũ��c(Na��)��c(CH3COO��)��c(ClO��)��c(OH��)��c(H��)��Dѡ����ȷ��
��ѡB��

 ��У����ϵ�д�
��У����ϵ�д�