��Ŀ����
20�������£�������������Һ��| �� | �� | �� | �� |
| 0.1mol•L-1 NaOH��Һ | pH=11 NaOH��Һ | 0.1mol•L-1CH3COOH��Һ | pH=3CH3COOH��Һ |
| A�� | ��ˮ�������c��H+�����ۣ��� | |
| B�� | ��ϡ�͵�ԭ����100����pH�����ͬ | |
| C�� | ����ۻ�ϣ�����ҺpH=7����V��NaOH����V��CH3COOH�� | |
| D�� | �ڢܻ�ϣ�����Һ�����ԣ���������Һ������Ũ�ȿ���Ϊc��CH3COO-����c��H+����c��Na+����c��OH-�� |
���� 0.1mol•L-1NaOH��Һ��0.1mol•L-1CH3COOH��Һ��Ƚϣ�CH3COOHΪ���ᣬ������ȫ���룬CH3COOH��Һˮ�ĵ���̶Ƚϴ���ϣ���Һ�ʼ��ԣ�
pH=11��NaOH��Һ��pH=3CH3COOH��Һ��Ƚϣ���CH3COOHΪ���ᣬ������ȫ���룬CH3COOH��ҺŨ�Ƚϴ��ߵ������ϣ������������Һ�����ԣ��Դ˽����⣮
��� �⣺A��CH3COOHΪ���ᣬ������ȫ���룬��Ũ��ʱ�������ˮ�ĵ�������ƽ�С����ˮ�������c��H+�����ۣ��٣���A��ȷ��
B.0.1mol•L-1CH3COOH��Һϡ�͵�ԭ����100����Ũ��Ϊ0.001mol/L�������Ϊ���ᣬ��pH��3����B����
C�������۵������ϣ�����ǿ�������Σ���Һ�ʼ��ԣ�����ҺpH=7�������Ӧ�Թ�������C����
D���ڢܻ�ϣ�����Һ�����ԣ���NaOH���٣�������c��CH3COO-����c��H+����c��Na+����c��OH-������D��ȷ��
��ѡAD��
���� ���⿼����������ʵĵ���ƽ�⣬Ϊ��Ƶ���㣬������ѧ���ķ��������Ŀ��飬��Ŀ������ǿ�ᡢ���ᡢǿ�������Һϡ�͡�pH�Ĵ�С�Ƚϣ�������ʵ���ƽ����ƶ����жϣ��ѶȲ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
13�����й���̼���ƣ�̼�����������������ʵıȽ���ȷ���ǣ�������
| A�� | ����ʱ��̼�����Ʊ�̼�������ֽ� | |
| B�� | ̼�������������̼���������մ� | |
| C�� | ̼��������Һ�����ԣ�̼������Һ�Լ��� | |
| D�� | �ֱ�������ʵ��������ְ�ɫ��ĩ�м���������Ũ�ȵ�ϡ���ᣬ������������ʣ�̼�����Ʊ�̼������ |
14�� ��ͼ��ʾ�ļס��ҡ����������ʾ�������ͬ��ij��Ԫ�أ���ͷ��ʾ���ʼ��ת��һ������ʵ�֣�������ǣ�������
��ͼ��ʾ�ļס��ҡ����������ʾ�������ͬ��ij��Ԫ�أ���ͷ��ʾ���ʼ��ת��һ������ʵ�֣�������ǣ�������
��C ��H2O2 ��Al ��Fe ��HNO3 ��H2S��
 ��ͼ��ʾ�ļס��ҡ����������ʾ�������ͬ��ij��Ԫ�أ���ͷ��ʾ���ʼ��ת��һ������ʵ�֣�������ǣ�������
��ͼ��ʾ�ļס��ҡ����������ʾ�������ͬ��ij��Ԫ�أ���ͷ��ʾ���ʼ��ת��һ������ʵ�֣�������ǣ���������C ��H2O2 ��Al ��Fe ��HNO3 ��H2S��
| A�� | �٢ڢۢ� | B�� | �ڢܢݢ� | C�� | �٢ۢݢ� | D�� | ȫ�� |
9����NA��ʾ�����ӵ�������ֵ��������������ȷ���ǣ�������
| A�� | ���³�ѹ�£�46 g�л���C2H6O�к��м��Լ�����Ŀһ��Ϊ7NA | |
| B�� | ��״���£�22.4 L���Ȼ�̼�������еĹ��ۼ���ĿΪ4NA | |
| C�� | ��״���£�5.6 L NO��5.6 L O2��ɵĻ������������ԭ����ΪNA | |
| D�� | ���³�ѹ�£�33.6 L������56 g����ַ�Ӧ��ת�Ƶĵ�����Ϊ3NA |
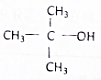
15�����й����л����˵����ȷ���ǣ�������
| A�� | ����ͱ����ܷ���������Ӧ��ȡ����Ӧ | |
| B�� | ��ϩ�;�����ϩ���ܷ����ӳɷ�Ӧ | |
| C�� | �Ҵ�����������ж����ǻ���������NaOH������Ӧ | |
| D�� | ���ۡ���ά��ˮ������ղ��ﻥΪͬ���칹�� |

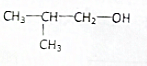
 H2CO3
H2CO3