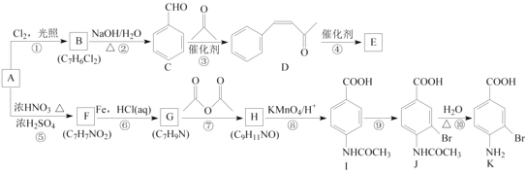
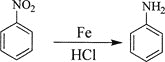
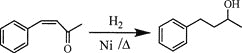
��Ŀ����
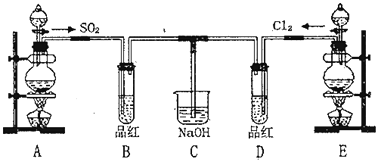
����Ŀ����ͼ��ʾ����A��F֮���ת����ϵ������AΪ����ɫ�������ʣ�B��CΪ��ɫ��Һ��DΪ���壬E��FΪ��ɫ����������д���и��գ�
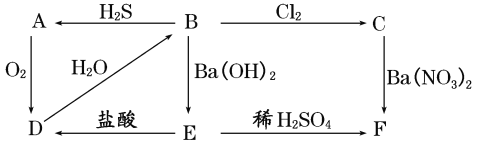
��1��д�������ʵĻ�ѧʽ��
AΪ______��BΪ_____��CΪ_____��DΪ_____��EΪ_____��FΪ______��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ��
B��A��________________________��
B��C��___________________________��
��3��д��C��F�����ӷ���ʽ��___________________��
��4����A��F���������У��������������л�ԭ�Ե���(����ĸ����)______��
���𰸡�S H2SO3 H2SO4 SO2 BaSO3 BaSO4 H2SO3��2H2S===3S����3H2O H2SO3��Cl2��H2O===H2SO4��2HCl SO42-��Ba2��===BaSO4�� ABDE
��������
AΪ����ɫ���壬��ת����ϵ��֪��AΪS����DΪSO2����ˮ��Ӧ���ɵ�BΪH2SO3�����л�ԭ�ԣ�������������������ԭ��Ӧ����CΪH2SO4��B������������Ӧ����EΪBaSO3��FΪBaSO4����϶�Ӧ���ʵ����ʷ������
(1)�������Ϸ�����֪��AΪS��BΪH2SO3��CΪH2SO4��DΪSO2��EΪBaSO3��FΪBaSO4���ʴ�Ϊ��S��H2SO3��H2SO4��SO2��BaSO3��BaSO4��
(2)B��A�Ļ�ѧ����ʽ��2H2S+H2SO3=3S��+3H2O��B��C�Ļ�ѧ����ʽ��Cl2+H2SO3+H2O=H2SO4+2HCl���ʴ�Ϊ��2H2S+H2SO3=3S��+3H2O��Cl2+H2SO3+H2O=H2SO4+2HCl��
(3)CΪH2SO4���������ᱵ��Ӧ�������ᱵ��������Ӧ�����ӷ���ʽΪBa2++SO42-�TBaSO4�����ʴ�Ϊ��Ba2++SO42-�TBaSO4����
(4)��A��F���������У��������������л�ԭ�ԣ�˵��SԪ�صĻ��ϼۼ������ߣ����ܽ��ͣ�SԪ�صĻ��ϼ۽����м��̬����S��H2SO3��SO2��BaSO3���ʴ�Ϊ��ABDE��