��Ŀ����
11�������Ǵ����о��εġ��˹��̵������·�����N2�ڴ���������ˮ������Ӧ��2N2��g��+6H2O��l��?4NH3��g��+3O2��g����H=+1530.4kJ/mol�����������գ���1��������Ӧ�ڸ��£�����¡����¡��κ��¶ȣ������Է����У�
��2���÷�Ӧƽ�ⳣ��K�ı���ʽK=$\frac{{c}^{4}��N{H}_{3}��•{c}^{3}��{O}_{2}��}{{c}^{2}��{N}_{2}��}$��
��3��������Ӧ�ﵽƽ����������������䣬�����¶ȣ����´ﵽƽ�⣬��ac��
a��ƽ�ⳣ��K���� b��H2O��Ũ�ȼ�С c�������ڵ�ѹǿ���� d��v����O2����С
��4���о�С��ֱ����ĸ��ݻ�Ϊ2�����ܱ������У�����N2 1mol��H2O 3mol���ڴ��������½��з�Ӧ3Сʱ��ʵ�����ݼ�����
| ��� | ��һ�� | �ڶ��� | ������ | ������ |
| t/�� | 30 | 40 | 50 | 80 |
| NH3������/��10-6mol�� | 4.8 | 5.9 | 6.0 | 2.0 |
��5����ˮ��ʵ���ҳ��õ����
����CaCl2��Һ��ͨ��CO2�����ͣ�������������ͨ��һ������NH3�������ɫ��������ʱ��Һ��һ���е�������NH4Cl�����õ���ƽ�����۽�������ʵ������H2CO3��Һ�е��������CO32-���٣����û�г��������백ˮ�ٽ�H2CO3�ĵ��룬CO32-����Ũ�������г���������
���������еμӰ�ˮ��������Һ�����ԣ�������Ũ�ȵĹ�ϵ��c��NH4+����c��OH-����c��Cl-����c��H+����
���� ��1�����ݡ�G=��H-T•��S�����жϣ����G��0����Ӧ���Է����У����G��0�������Է����У�
��2������ƽ�ⳣ������Ķ��壬������Ũ����֮�����Ϸ�Ӧ��Ũ����֮����
��3��a������ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�ⳣ��K����
b�������¶ȣ�H2O��Ũ�Ȳ��䣻
c�������¶ȣ�ƽ�������ƶ��������ڵ�ѹǿ����
d�������¶����淴Ӧ���ʶ����ӣ�
��4��3Сʱ����NH3Ũ�ȵı仯������ʾ���������¶���ߵ���ƽ��ʱ�����������ʵ�����С���������¶ȶԴ����Ļ��Ե�Ӱ�죻
��5����ͨ��һ������NH3��ʹ��Һ�ʼ��ԣ��ܹ�����NH4Cl��
�ڸ��ݵ���غ㣬����Һ�����ԣ�c��H+��=c��OH-��������c ��C1-��=c��NH4+������ϵΪNH4Cl��Һ��NH3��H2O����ˮ�����϶�ʱ����Һ�ʼ��ԣ�c��NH4+����c��OH-����c��Cl-����c��H+����
��� �⣺��1��2N2��g��+6H2O��l��?4NH3��g��+3O2��g����H=+1530.4kJ/mol����Ӧ�С�H��0����S��0�����Ա����ڸ��µ������²����Է����У��ʴ�Ϊ�����£�
��2����Ӧ2N2��g��+6H2O��l��?4NH3��g��+3O2��g����ƽ�ⳣ������ʽΪ��K=$\frac{{c}^{4}��N{H}_{3}��•{c}^{3}��{O}_{2}��}{{c}^{2}��{N}_{2}��}$���ʴ�Ϊ��K=$\frac{{c}^{4}��N{H}_{3}��•{c}^{3}��{O}_{2}��}{{c}^{2}��{N}_{2}��}$��
��3��a������ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�ⳣ��K����ѡ��
b�������¶ȣ�H2O��Ũ�Ȳ��䣬�ʲ�ѡ��
c�������¶ȣ�ƽ�������ƶ��������ڵ�ѹǿ����ѡ��
d�������¶����淴Ӧ���ʶ����ӣ��ʲ�ѡ��
�ʴ�Ϊ��ac��
��4��3Сʱ����NH3Ũ�ȵı仯����v��NH3��=$\frac{\frac{2��1{0}^{-6}mol-0}{2L}}{3h}$=33��10-7mol/L•h���������¶���ߵ���ƽ��ʱ�����������ʵ�����С�������Ǵ�����80����Լ�С����Ӧ���ʷ����������ʴ�Ϊ��33��10-7mol/L•h��������80����Լ�С����Ӧ���ʷ���������
��5����ͨ��һ������NH3��ʹ��Һ�ʼ��ԣ��ܹ�����NH4Cl������H2CO3��Һ�е��������CO32-���٣����û�г��������백ˮ�ٽ�H2CO3�ĵ��룬CO32-����Ũ�������г����������ʴ�Ϊ��NH4Cl������H2CO3��Һ�е��������CO32-���٣����û�г��������백ˮ�ٽ�H2CO3�ĵ��룬CO32-����Ũ�������г���������
����ϵΪNH4Cl��Һ��NH3��H2O����ˮ�����϶�ʱ����Һ�ʼ��ԣ�c��NH4+����c��OH-����c��Cl-����c��H+����
�ʴ�Ϊ��c��NH4+����c��OH-����c��Cl-����c��H+����
���� ���⿼�黯ѧƽ�ⳣ������ʽ����д���Լ�Ӱ�컯ѧ���ʵ����غ�������ʵ�ʵ��̽�������ü��跨��������ɣ��ѶȲ���

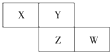 X��Y��Z��Ϊ������Ԫ�أ�X�ĵ����ڿ����к�����ߣ����������ڱ���λ����ͼ��������˵������ȷ���ǣ�������
X��Y��Z��Ϊ������Ԫ�أ�X�ĵ����ڿ����к�����ߣ����������ڱ���λ����ͼ��������˵������ȷ���ǣ�������| A�� | X����̬�⻯���Y���ȶ� | |
| B�� | W������������Ӧˮ��������Ա�Z��ǿ | |
| C�� | Z�ķǽ����Ա�Y���� | |
| D�� | X��Y�γɵĻ����ﶼ������ˮ |
| A�� | ��������ͭ�ֱ�������ϡ�����Ũ�����ַ�Ӧ��������������ʵ�����ͬ | |
| B�� | �����������ֱ����������������ַ�Ӧ���������ʵ����ʵ�����ͬ | |
| C�� | �����������ֱ�����������������Һ�������ַ�Ӧ��������������һ����ͬ | |
| D�� | �������������ֱ��������������ƺ����Ƴ�ַ�Ӧ��ת�Ƶĵ�������ͬ |
 ��֪�ס��ҡ�����������ѧ��ѧ���������ʣ�һ�������¿ɷ�����ͼת��������������ӷ���ʽ��ѧ����ʽ������ǣ�������
��֪�ס��ҡ�����������ѧ��ѧ���������ʣ�һ�������¿ɷ�����ͼת��������������ӷ���ʽ��ѧ����ʽ������ǣ�������| A�� | �ٿ���ΪCH4+Cl2$\stackrel{��}{��}$CH3Cl+HCl | B�� | �ٿ���ΪFe+2Fe3+�T3Fe2+ | ||
| C�� | �ڿ���Ϊ2NO+O2�T2NO2 | D�� | �ڿ���ΪCO32-+H2O+CO2�T2HCO3- |
| A�� | ���õķ�ɢϵ���ڽ��壬�ɷ��������ЧӦ | |
| B�� | ���õķ�ɢϵ�У���ɢ�ʵ���Ҫ�ɷ�ΪFeO | |
| C�� | �÷�ɢϵ���е�Ӿʵ��ʱ��������Χ��ɫ���� | |
| D�� | ��������ˮ��Һ�еμ�Ũ��ˮ������������ |
| A�� |  ��ȡ���� | B�� |  ʢװNaOH��Һ | C�� | 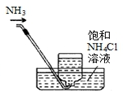 �ռ����� | D�� |  ��ȥ����CO2���� |
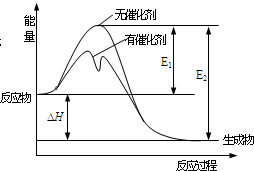 2SO2��g��+O2��g��?2SO3��g����Ӧ�����������仯��ͼ��ʾ��ͼ��E1��ʾ����Ӧ�Ļ�ܣ�E2��ʾ�淴Ӧ�Ļ�ܣ��������й�������ȷ���ǣ�������
2SO2��g��+O2��g��?2SO3��g����Ӧ�����������仯��ͼ��ʾ��ͼ��E1��ʾ����Ӧ�Ļ�ܣ�E2��ʾ�淴Ӧ�Ļ�ܣ��������й�������ȷ���ǣ�������| A�� | �÷�ӦΪ���ȷ�Ӧ | |
| B�� | �����¶ȣ���Ӱ�����Ӱٷ��� | |
| C�� | ʹ�ô���ʹ�÷�Ӧ�ķ�Ӧ�ȷ����ı� | |
| D�� | E1-E2=��H |
| A�� | 7.8 g Na2O2�к��е���������ĿΪ0.2NA | |
| B�� | ��״���£�2.24 L CHCl3�ķ�����Ϊ0.1NA | |
| C�� | 1 L 0.1 mol/L Al2��SO4��3��Һ�У�Al3+����ĿΪ0.2NA | |
| D�� | 0.1 mol Fe������ϡHNO3��Ӧ��ת�Ƶ�����Ϊ0.3NA |
| A�� | AlCl3��Һ��ͨ������İ�ˮ��Al3++4NH3•H2O=AlO2-+2H2O+4NH4+ | |
| B�� | ��NH4HCO3 ��Һ�мӹ���NaOH ��Һ�����ȣ�NH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O | |
| C�� | ��CuƬ����ϡ�����У�3Cu+8H++2NO3-=3Cu2++2NO��+4H2O | |
| D�� | ��Na2S2O3��Һ�м���ϡ���2S2O32-+2H+=SO42-+3S��+H2O |