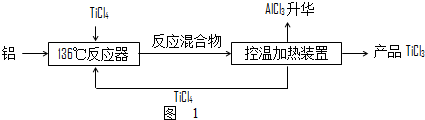
��Ŀ����
12����þ���仯�����ڹ�ũҵ������Ӧ�ù㷺����֪����þ����Ҫ�ɷ�ΪMg2B2O5•H2O����ɰ�Ļ�ѧʽΪNa2B4O7•10H2O��������þ����ȡ����þ������Ĺ�������Ϊ��
�ش������й����⣺
��1����ɰ������ˮ����H2SO4��pH2��3��ȡH3BO3����Ӧ�����ӷ���ʽΪB4O72-+2H++5H2O=4H3BO3��XΪH3BO3���������ˮ�IJ������Mg��ȡ����Ļ�ѧ����ʽΪMg+B2O3�T2B+3MgO��
��2��MgCl2•7H2O��Ҫ��HCl��Χ�м��ȣ���Ŀ���Ƿ�ֹMgCl2ˮ������Mg��OH��2�����ö��Ե缫���MgCl2��Һ����������ӦʽΪMg2++2H2O+2e-=Mg��OH��2��+H2����
��3��þ-H2O2����ȼ�ϵ�صķ�Ӧ����ΪMg+H2O2+2H+�TMg2++2H2O����������ӦʽΪH2O2+2H++2e-=2H2O������ʼ�������ҺpH=1����pH=2ʱ��Һ��Mg2+����Ũ��Ϊ0.045mol•L-1����֪Ksp[Mg��OH��2]=5.6��10-12������ҺpH=6ʱû�� ����С���û�С���Mg��OH��2����������
���� ��þ����Ҫ�ɷ�ΪMg2B2O5•H2O����ɰ�Ļ�ѧʽΪNa2B4O7•10H2O��������þ����ȡ����þ������Ĺ�����������þ�������������Ũ��Һ���˵õ�����Ϊ������þ������Ũ�����ܽ�ͨ������Ũ���õ��Ȼ�þ�ᾧˮ������Ȼ��������м��ȵõ��Ȼ�þ���壬�����õ�þ����Һ����Ҫ��NaBO2��ͨ������������̼����õ���ɰ��������ˮ����H2SO4��pH2��3��ȡH3BO3�����ȵõ�B2O3��
��1����H2SO4��pH2��3����ɰ�е�Na2B4O7������Һ������H3BO3 ��XΪH3BO3���������ˮ�IJ����ж�ΪB2O3����Mg��Ӧ���ɴ��������þ��
��2���Ȼ�þ��ˮ��Һ��ˮ������������þ�����Ե缫���MgCl2��Һ���������ӵõ���������������ˮ�ĵ���ƽ���ƻ���ˮ������������������Ũ������þ�����γ�������þ�������ϲ�д���缫��Ӧ��
��3��ȼ�ϵ�����������ǹ�������õ���������ˮ�����ݵ������ҺPH�仯��ϵ�ط�Ӧ����þ����Ũ�ȣ�PH=6��������������Ũ�ȣ��������þ����Ũ�ȼ���Ũ���̺��ܶȻ������ȽϷ����Ƿ�����������þ������
��� �⣺��1����H2SO4��pH2��3����ɰ�е�Na2B4O7������Һ������H3BO3 ����Ӧ�����ӷ���ʽΪ��B4O72-+2H++5H2O=4H3BO3��XΪH3BO3���������ˮ�IJ����ж�ΪB2O3����Mg��Ӧ���ɴ��������þ����Ӧ�Ļ�ѧ����ʽΪ3Mg+B2O3$\frac{\underline{\;����\;}}{\;}$2B+3MgO��
�ʴ�Ϊ��B4O72-+2H++5H2O=4H3BO3��3Mg+B2O3$\frac{\underline{\;����\;}}{\;}$2B+3MgO��
��2��MgCl2•7H2O��Ҫ��HCl��Χ�м��ȣ���Ϊ�˷�ֹ�Ȼ�þˮ������������þ�����ö��Ե缫���MgCl2��Һ�����������ӵõ���������������ˮ�ĵ���ƽ���ƻ���ˮ������������������Ũ������þ�����γ�������þ�������ϲ�д��������ӦʽΪ��2H2O+Mg2++2e-=H2��+Mg��OH��2����
�ʴ�Ϊ����ֹMgCl2ˮ������Mg��OH��2��2H2O+Mg2++2e-=H2��+Mg��OH��2����
��3��þ-H2O2����ȼ�ϵ�صķ�Ӧ����ΪMg+H2O2+2H+�TMg2++2H2O���������ǹ�������õ���������ˮ�ķ�Ӧ��������ӦʽH2O2+2H++2e-=2H2O������ʼ�������ҺpH=1����pH=2ʱ��Һ�У�������Ũ�ȼ�С0.1mol/L-0.01mol/L=0.09mol/L�����ݷ�Ӧ����ʽ�õ�Mg2+����Ũ��=0.045mol/L��Ksp[Mg��OH��2]=5.6��10-12������ҺpH=6ʱ��c��OH-��=10-8mol/L����Qc=c��Mg2+����c2��OH-��=0.045mol/L��10-16mol/L=4.5��10-18��Ksp[Mg��OH��2]��˵����������þ�������ɣ�
�ʴ�Ϊ��H2O2+2H++2e-=2H2O��0.045 mol•L-1��û�У�
���� ���⿼���������ˮ�⡢ԭ���ԭ���͵���ԭ���ķ����������ܽ�ƽ��ļ���Ӧ�ã���Ŀ�Ѷ��Դ������ѵ㣬����ʱҪ���������Ŀ����������ϵ�ͱ������ݣ�����������ϵ�����������ĸ�����ٽ��⣮

 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�| A�� | 1L 1mol•L-1��CH3COOH��Һ�к���NA�������� | |
| B�� | ���ʵ���Ũ��Ϊ0.5 mol•L-1��MgCl2��Һ�У�����Cl-����ΪNA | |
| C�� | 5.6 g�����������ᷴӦת�Ƶĵ�����Ϊ0.3NA | |
| D�� | 1g O2��1g O3�������еĵ�������Ϊ0.5NA |

�״������Ѻ�3��5-�����������ӵIJ����������ʼ�����
| ���� | �е�/�� | �۵�/�� | �ܶȣ�20�棩/g•cm-3 | �ܽ��� |
| �״� | 64.7 | / | 0.7915 | ������ˮ |
| ���� | 34.5 | / | 0.7138 | ����ˮ |
| 3��5-������������ | / | 33��36 | / | �����ڼ״������ѣ�����ˮ |
�ٷ�����״��IJ����ǵ�����
����ȡ�õ��ķ�Һ©��ʹ��ǰ�����©��ϴ������Һʱ�л����ڷ�Һ©����������ϡ����¡����㣮
��2������õ����л��������ñ���NaHCO3��Һ������ʳ��ˮ����������ˮ����ϴ�ӣ��ñ���NaHCO3��Һϴ�ӵ�Ŀ���Ǣٳ�ȥHCl���ñ���ʳ��ˮϴ�ӵ�Ŀ���Ǣڳ�ȥ����NaHCO3�Ҽ��ٲ�����ʧ��
��3��ϴ����ɺ�ͨ�����²������롢�ᴿ�����ȷ�IJ���˳����dcab������ĸ����
a�������ȥ���� b���ؽᾧ c�����˳�ȥ����� d��������ˮCaCl2����
��4����Ӧ���ܳ��ֵĸ�����Ľṹ��ʽΪ
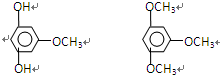 ��
�� 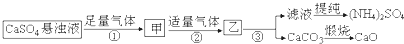
�����ƶ�����ȷ���ǣ�������
| A�� | ����ٺ͢��е��������ڿ�ѭ��ʹ�õ����� | |
| B�� | ������з����ķ�ӦΪ��Ca2++CO2+2NH3•H2O�TCaCO3��+2NH4++H2O | |
| C�� | ������ͨCO2�����ڣ�NH4��2SO4���� | |
| D�� | �����漰�Ļ�ѧ��Ӧ����������ԭ��Ӧ |
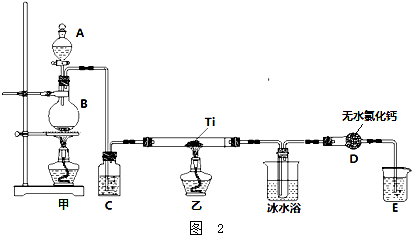
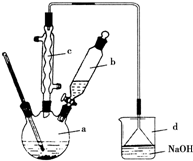 ʵ���Һϳ��屽��װ��ͼ���й��������£������кϳɲ���ش�
ʵ���Һϳ��屽��װ��ͼ���й��������£������кϳɲ���ش�| �� | �� | �屽 | |
| �ܶ�/g��cm3 | 0.88 | 3.10 | 1.50 |
| �е�/�� | 80 | 59 | 156 |
| ˮ���ܽ�� | �� | �� | �� |
��2����a�м���15mL��ˮ����������м����b��С�ļ���4.0mLҺ̬�壮��a�е��뼸���壬�а�������������Ϊ������HBr���壮�����μ���Һ����꣮д��a�з�����Ӧ�Ļ�ѧ����ʽ��2Fe+3Br2�T2FeBr3
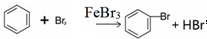 ��
����3��Һ�����������в�������ᴿ��
����a�м���10mLˮ��Ȼ����˳�ȥδ��Ӧ����м��
����Һ������10mLˮ��8mL10%��NaOH��Һ��10mLˮϴ�ӣ�NaOH��Һϴ�ӵ������dz�ȥHBr��δ��Ӧ��Br2��
����ֳ��Ĵ��屽�м�����������ˮ�Ȼ��ƣ����á����ˣ�������ˮ�Ȼ��Ƶ�Ŀ���Ǹ��
��4�������Ϸ���������屽�л����е���Ҫ����Ϊ����Ҫ��һ���ᴿ������еIJ�������Ϊ����
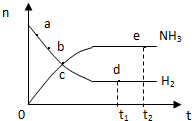
| A�� | ��a��������Ӧ���ʱȵ�b���Ĵ� | |
| B�� | ��c��������Ӧ�������淴Ӧ������� | |
| C�� | ��d��t1ʱ�̣���n��N2���ȵ�e��t2ʱ�̣�����n��N2���� | |
| D�� | �����������䣬773K�·�Ӧ��t1ʱ�̣���ʱ����������ƽ����ľ��뽫Ҫ��С |
| A�� | 1molH2 | B�� | 2 molNa2CO3 | C�� | 0.5mol�� | D�� | 2 molOH- |