ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΒΣΦΑΤδΜ·ΚœΈο‘ΎΙΛ≈©“Β…ζ≤ζ÷–ΨΏ”–÷Ί“ΣΉς”ΟΓΘ
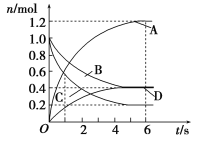
Θ®1Θ©Ρ≥–ΓΉιΫχ––ΙΛ“ΒΚœ≥…Α±N2Θ®gΘ©+3H2Θ®gΘ©2NH3Θ®gΘ©+QΒΡΡΘΡβ―–ΨΩΘ§‘Ύ1LΟή±’»ίΤς÷–Θ§Ζ÷±πΦ”»κ0.1molN2ΚΆ0.3molH2ΓΘ Β―ιΔΌΓΔΔΎΓΔΔέ÷–cΘ®N2Θ©Υφ ±ΦδΘ®tΘ©ΒΡ±δΜ·»γΆΦΥυ ΨΘ§ Β―ιΔΎ¥”ΩΣ ΦΒΫ¥οΤΫΚβΉ¥Χ§ΒΡΙΐ≥Χ÷–Θ§”ΟH2±μ ΨΒΡΤΫΨυΖ¥”ΠΥΌ¬ ΈΣ____ΓΘ”κ Β―ιΔΌœύ±»Θ§ Β―ιΔΎΥυ≤…”ΟΒΡ Β―ιΧθΦΰΩ…ΡήΈΣ____Θ®ΧνΉ÷ΡΗΘ©
aΘ°‘ω¥σ―Ι«Ω bΘ°Φθ–Γ―Ι«Ω cΘ°…ΐΗΏΈ¬Ε» dΘ°ΫΒΒΆΈ¬Ε» eΘ° Ι”Ο¥ΏΜ·ΦΝ

Θ®2Θ©NH3”Ο”Ύ¥ΠάμΖœΤχ÷–ΒΡΒΣ―θΜ·ΈοΘ§ΤδΖ¥”Π‘≠άμΈΣ2NH3Θ®gΘ©+NOΘ®gΘ©+NO2Θ®gΘ©2N2Θ®gΘ©+3H2OΘ®gΘ©+QΘ§”ϊΧαΗΏΖœΤχ÷–ΒΣ―θΜ·ΈοΒΡΉΣΜ·¬ Θ§Ω…≤…»ΓΒΡ¥κ © «____Θ®ΧνΉ÷ΡΗΘ©ΘΜ
aΘ°…ΐΗΏΈ¬Ε» bΘ°‘ω¥σ―Ι«Ω cΘ°‘ω¥σNH3ΒΡ≈®Ε»
ΓΨ¥πΑΗΓΩ0.012molL-1min-1 e c
ΓΨΫβΈωΓΩ
(1)ΗυΨίv=![]() ΦΤΥψΖ¥”ΠΥΌ¬ Θ§ΗυΨίΆΦœσΩ…÷ΣΔΎΒΫ¥οΤΫΚβΒΡ ±Φδ±»ΔΌΕΧΘ§ΒΫ¥οΤΫΚβ ±N2ΒΡ≈®Ε»”κΔΌœύΆ§Θ§Μ·―ßΤΫΚβ≤Μ“ΤΕ·Θ§Ι ΔΎ”κΔΌœύ±»Φ”ΝΥ¥ΏΜ·ΦΝΘ§ΔΌΚΆΔέ±»ΫœΩ…÷ΣΘ§ΔέΒΡΥΌ¬ ±»ΔΌ–ΓΘ§ΤΫΚβ ±ΒΣΤχΒΡ≈®Ε»ΗΏΘ§Φ¥ΤΫΚβΡφœρ“ΤΕ·Θ§Ι ΔέΦθ–ΓΝΥ―Ι«ΩΘΜ
ΦΤΥψΖ¥”ΠΥΌ¬ Θ§ΗυΨίΆΦœσΩ…÷ΣΔΎΒΫ¥οΤΫΚβΒΡ ±Φδ±»ΔΌΕΧΘ§ΒΫ¥οΤΫΚβ ±N2ΒΡ≈®Ε»”κΔΌœύΆ§Θ§Μ·―ßΤΫΚβ≤Μ“ΤΕ·Θ§Ι ΔΎ”κΔΌœύ±»Φ”ΝΥ¥ΏΜ·ΦΝΘ§ΔΌΚΆΔέ±»ΫœΩ…÷ΣΘ§ΔέΒΡΥΌ¬ ±»ΔΌ–ΓΘ§ΤΫΚβ ±ΒΣΤχΒΡ≈®Ε»ΗΏΘ§Φ¥ΤΫΚβΡφœρ“ΤΕ·Θ§Ι ΔέΦθ–ΓΝΥ―Ι«ΩΘΜ
(2)ΧαΗΏΖœΤχ÷–ΒΣ―θΜ·ΈοΒΡΉΣΜ·¬ Θ§”ΠΗΡ±δΧθΦΰ ΙΤΫΚβœρ’ΐΖ¥”Π“ΤΕ·Θ§ΫαΚœΤΫΚβ“ΤΕ·‘≠άμΖ÷ΈωΓΘ
(1)ΗυΨίΆΦœσΩ…÷ΣΘ§ΔΎ‘Ύ10min ±¥οΒΫΤΫΚβΘ§¥Υ ±ΒΣΤχΒΡ≈®Ε»±δΜ·ΈΣ0.04mol/LΘ§ΗυΨίΖΫ≥Χ ΫΩ…÷ΣΘ§Α±ΤχΒΡ≈®Ε»±δΜ·ΝΥ0.08mol/LΘ§ΗυΨίv=![]() Ω…÷Σv(NH3)=
Ω…÷Σv(NH3)=![]() =0.008molL-1min-1Θ§‘ρv(H2)=0.012molL-1min-1Θ§ΗυΨίΆΦœσΩ…÷ΣΔΎΒΫ¥οΤΫΚβΒΡ ±Φδ±»ΔΌΕΧΘ§ΒΫ¥οΤΫΚβ ±N2ΒΡ≈®Ε»”κΔΌœύΆ§Θ§Μ·―ßΤΫΚβ≤Μ“ΤΕ·Θ§Ι ΔΎ”κΔΌœύ±»Φ”ΝΥ¥ΏΜ·ΦΝΘ§Ι ¥πΑΗΈΣeΘΜ
=0.008molL-1min-1Θ§‘ρv(H2)=0.012molL-1min-1Θ§ΗυΨίΆΦœσΩ…÷ΣΔΎΒΫ¥οΤΫΚβΒΡ ±Φδ±»ΔΌΕΧΘ§ΒΫ¥οΤΫΚβ ±N2ΒΡ≈®Ε»”κΔΌœύΆ§Θ§Μ·―ßΤΫΚβ≤Μ“ΤΕ·Θ§Ι ΔΎ”κΔΌœύ±»Φ”ΝΥ¥ΏΜ·ΦΝΘ§Ι ¥πΑΗΈΣeΘΜ
(2)ΧαΗΏΖœΤχ÷–ΒΣ―θΜ·ΈοΒΡΉΣΜ·¬ Θ§”ΠΗΡ±δΧθΦΰ ΙΤΫΚβœρ’ΐΖ¥”Π“ΤΕ·ΘΜ
aΘ°ΗΟΖ¥”Π’ΐΖ¥”Π «Ζ≈»»Ζ¥”ΠΘ§…ΐΗΏΈ¬Ε»Θ§ΤΫΚβœρΡφΖ¥”Π“ΤΕ·Θ§ΒΣ―θΜ·ΈοΒΡΉΣΜ·¬ ΫΒΒΆΘ§Ι a¥μΈσΘΜ
bΘ°ΗΟΖ¥”Π’ΐΖ¥”Π «ΧεΜΐ‘ω¥σΒΡΖ¥”ΠΘ§‘ω¥σ―Ι«ΩΘ§ΤΫΚβœρΡφΖ¥”Π“ΤΕ·Θ§ΒΣ―θΜ·ΈοΒΡΉΣΜ·¬ ΫΒΒΆΘ§Ι b¥μΈσΘΜ
cΘ°‘ω¥σNH3ΒΡ≈®Ε»Θ§ΤΫΚβœρ’ΐΖ¥”Π“ΤΕ·Θ§ΒΣ―θΜ·ΈοΒΡΉΣΜ·¬ ‘ω¥σΘ§Ι c’ΐ»ΖΘΜ
Ι ¥πΑΗΈΣcΓΘ

ΓΨΧβΡΩΓΩ»γ±μ «ΦΗ÷÷≥ΘΦϊ»θΥαΒΡΒγάκΤΫΚβ≥Θ ΐ(25Γφ)ΓΘ
Υα | ΒγάκΖΫ≥Χ Ϋ | ΒγάκΤΫΚβ≥Θ ΐK |
CH3COOH | CH3COOH | 1.76ΓΝ10-5 |
H2CO3 | H2CO3 | K1=4.4ΓΝ10-7 |
HCO | K2=4.7ΓΝ10-11 | |
H2S | H2S | K1=1.3ΓΝ10-7 |
HS- | K2=7.1ΓΝ10-15 | |
H3PO4 | H3PO4 | K1=7.1ΓΝ10-3 |
H2PO4- | K2=6.3ΓΝ10-8 | |
HPO42- | K3=4.2ΓΝ10-13 |
(1) Β±Έ¬Ε»…ΐΗΏ ±Θ§K÷Β_____(ΧνΓΑ‘ω¥σΓ±ΓΑΦθ–ΓΓ±ΜρΓΑ≤Μ±δΓ±)ΓΘ
(2) »τΑ―CH3COOHΓΔH2CO3ΓΔHCO3-ΓΔH2SΓΔHS-ΓΔH3PO4ΓΔH2PO4-ΓΔHPO42-ΕΦΩ¥≥…ΥαΘ§Τδ÷–Υα–‘Ήν«ΩΒΡ «_____Θ§Ήν»θΒΡ «_____ΓΘ”…άκΉ”Ζ¥”ΠΙφ¬…ΦΑ…œ ω ΐΨί≈–ΕœΘ§œ¬Ν–ΗςΉιΈο÷ ‘Ύ»ή“Κ÷–ΜλΚœΚσΡήΖΔ…ζΖ¥”ΠΒΡ”–_____(ΧνΉ÷ΡΗ)ΓΘ
a. CH3COOH+NaH2PO4
b. H2S+Na2CO3
c. CO2+Na2HPO4
(3) Ζ÷ΈωΕύ‘Σ»θΥαΗς≤ΫΒγάκΒΡK÷Β¥σ–ΓΘ§Ρψ»œΈΣ“ΜΕ®≈®Ε»ΒΡΡ≥Εύ‘Σ»θΥα»ή“Κ÷–Θ§c(H+)ΒΡ¥σ–Γ÷ς“Σ”…_______ΨωΕ®ΓΘΦΌ…ηΡ≥«βΝρΥα»ή“ΚΒΡ≈®Ε»ΈΣ0.001 3 molΓΛL-1Θ§‘ρ»ή“Κ÷–c(H+)=______ΓΘ
(4) «κ…ηΦΤ“Μ÷÷ΖΫΑΗΘ§ΡήΆ®Ιΐ÷±ΙέΒΡ Β―ιœ÷œσ≈–Εœ¥ΉΥαΒΡΥα–‘«Ω”ΎΧΦΥαΘ§ΗΟΖΫΑΗΥυ”Ο ‘ΦΝ «____Θ§Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ_____ΓΘ
ΓΨΧβΡΩΓΩ‘ΎΦΉΓΔ““ΓΔ±ϊ»ΐΗω≤ΜΆ§Οή±’»ίΤς÷–Α¥≤ΜΆ§ΖΫ ΫΆΕΝœΘ§“ΜΕ®ΧθΦΰœ¬ΖΔ…ζΖ¥”ΠΘ®Τπ ΦΈ¬Ε»ΚΆΤπ ΦΧεΜΐœύΆ§Θ©ΘΚA2(g)+3B2(g)![]() 2AB3(g) ΠΛH<0Θ§œύΙΊ ΐΨί»γœ¬±μΥυ ΨΘΚ
2AB3(g) ΠΛH<0Θ§œύΙΊ ΐΨί»γœ¬±μΥυ ΨΘΚ
»ίΤς | ΦΉ | ““ | ±ϊ |
œύΙΊΧθΦΰ | ΚψΈ¬Κψ»ί | Ψχ»»Κψ»ί | ΚψΈ¬Κψ―Ι |
Ζ¥”ΠΈοΆΕΝœ | 1mol A2ΓΔ3molB2 | 2molAB3 | 2mol AB3 |
Ζ¥”ΠΈοΒΡΉΣΜ·¬ | aΦΉ | a““ | a±ϊ |
Ζ¥”ΠΒΡΤΫΚβ≥Θ ΐK= | KΦΉ | K““ | K±ϊ |
ΤΫΚβ ±AB3ΒΡ≈®Ε»/molΓΛL-1 | cΦΉ | c““ | c±ϊ |
ΤΫΚβ ±AB3ΒΡΖ¥”ΠΥΌ¬ /molΓΛL-1ΓΛmin-1 | vΦΉ | v““ | v±ϊ |
œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «
A. vΦΉ=v±ϊ B. c““ΘΦ c±ϊ C. aΦΉ +a““ΘΦ1 D. K““ΓήK±ϊ