��Ŀ����
����Ŀ���������߷ֱ��ʾԪ�ص�ij��������˵�����Ĺ�ϵ(ZΪ�˵������YΪԪ�ص��й�����)��
(1)��������Ԫ���й�������������߱��������Ӧ�Ŀո���:
a. 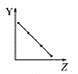 b.
b. 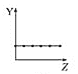 c.
c. 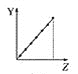 d.
d. 
�ٵڢ�A��Ԫ�صļ۵�����________��
�ڵ�������Ԫ�ص�����ϼ�________��
��F-��Na+��Mg2+��Al3+�����Ӱ뾶________��
(2)Ԫ��X��Y��Z��M��N��Ϊ����������Ԫ�أ���ԭ����������������֪Yԭ������������������������֮��Ϊ3��4��MԪ��ԭ�ӵ���������������Ӳ���֮��Ϊ4��3��N-��Z+��X+�İ뾶��С��������XN������Ϊ���塣�ݴ˻ش�
��XΪ___________(����)��YΪ____________(Ԫ�ط���)��Zԭ�ӽṹʾ��ͼΪ________________��
��N������������ˮ����Ļ�ѧʽΪ________________��
��M�����������Ļ�ѧʽΪ________________________��
���𰸡�b c a �� O ![]() HClO4 SiO2
HClO4 SiO2
��������
��1���ٸ���ͬһ����Ԫ�ص�������������ȣ�
�ڵ�3���ڵ�����ϼ۴��������������ߣ�
��������������ͬ�ĵ��Ӳ�ṹ���˵����ԽС�����Ӱ뾶Խ��
��2��Ԫ��X��Y��Z��M��N��Ϊ����������Ԫ�أ���ԭ����������������֪Yԭ������������������������֮��Ϊ3��4����Y������������Ϊ6�����������Ϊ8���ϣ���YΪOԪ�أ�MԪ��ԭ�ӵ���������������Ӳ���֮��Ϊ4��3��MΪ�������ڵ�SiԪ�أ�N-��Z+��X+�İ뾶��С����֪NΪCl��ZΪNa��XΪH��������HCl������Ϊ���壬�Դ������
��1������A��Ԫ�ص�������������ȣ�ͼ��b���ϣ��ʴ�Ϊ��b��
�ڵ�3���ڵ�����ϼ۴��������������ߣ�ͼ��c���ϣ��ʴ�Ϊ��c��
��������������ͬ�ĵ��Ӳ�ṹ���˵����ԽС�����Ӱ뾶Խ�����Ӱ뾶��F-��Na+��Mg2+��Al3+��ͼ��a���ϣ��ʴ�Ϊ��a��
��2����������������֪��XΪ�⣬YΪO��Z��ԭ�ӽṹʾ��ͼΪ ���ʴ�Ϊ���⣻O��
���ʴ�Ϊ���⣻O�� ��
��
��N������������ˮ����Ļ�ѧʽΪHClO4���ʴ�Ϊ��HClO4��
��MΪSi�������������SiO2���ʴ�Ϊ��SiO2��

 ������ÿ�ʱ�Ż���ҵϵ�д�
������ÿ�ʱ�Ż���ҵϵ�д�����Ŀ����ҵ�Ͽ���һ����̼�ϳɿ�������Դ�״���
(1)��֪����.3CO(g)��6H2(g) ![]() CH3CH��CH2(g)��3H2O(g) ��H1��-301.3kJ/mol��
CH3CH��CH2(g)��3H2O(g) ��H1��-301.3kJ/mol��
��.3CH3OH(g) ![]() CH3CH��CH2(g)��3H2O(g) ��H2��-31.0kJ/mol��
CH3CH��CH2(g)��3H2O(g) ��H2��-31.0kJ/mol��
��CO��H2�ϳ���̬�״����Ȼ�ѧ����ʽΪ___________________________________
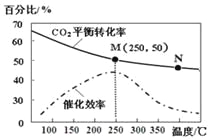
(2)ij����С����Cu2O/ZnO�������������£���500��ʱ���о���n(H2)��n(CO)�ֱ�Ϊ2��1��5��2ʱCO��ת���ʱ仯���(��ͼ1��ʾ)����ͼ�б�ʾn(H2)��n(CO)��2��1�ı仯����Ϊ________(��������a����������b��)��ԭ����_________________��

(3)ij����С�����ܱ������г���һ������CO��H2�ϳ���̬�״����ֱ���A��B���ֲ�ͬ���������·�����Ӧ��һ��ʱ�����CH3OH�IJ������¶ȵĹ�ϵ��ͼ2��ʾ������˵����ȷ����____________(��ѡ����ĸ)��
a.ʹ�ô���A�ܼӿ���ػ�ѧ��Ӧ���ʣ�������A��δ���뷴Ӧ
b.�ں��º�ѹ��ƽ����ϵ�г��������CH3OH�IJ��ʽ���
c.��2v(CO)����v(H2)��ʱ����Ӧ�ﵽƽ��״̬
(4)һ���¶��£����ݻ���Ϊ2L�����������ܱ������У������·�ʽ���뷴Ӧ�һ��ʱ���ﵽƽ�⡣
���� | �� | �� |
��Ӧ����ʼͶ���� | 2 mol CO��6 mol H2 | a mol CO��b mol H2��c mol CH3OH(g)(a��b��c����Ϊ��) |
��������ƽ��������ѹǿΪ��ʼʱ��![]() ������¶��£��÷�Ӧ��ƽ�ⳣ��K��_______��Ҫʹƽ��������������������ͬ��ֵ����������ȣ�����ʼʱά�ֻ�ѧ��Ӧ���淴Ӧ������У�����������c��ȡֵ��ΧΪ_____________________________________��
������¶��£��÷�Ӧ��ƽ�ⳣ��K��_______��Ҫʹƽ��������������������ͬ��ֵ����������ȣ�����ʼʱά�ֻ�ѧ��Ӧ���淴Ӧ������У�����������c��ȡֵ��ΧΪ_____________________________________��
(5)CO���ճ�����������ء�

���������β����CO����������CO�����ǣ�����ԭ��������ȼ�ϵ�أ����е������������(Y2O3)�������(ZrO2)���壬�ܴ���O2�������ĵ缫��ӦʽΪ__________________��
��̼�������[(CH3O)2CO]����С����һ����ɫ������Ʒ����CO�ϳ�(CH3O)2CO����绯ѧ�ϳ�ԭ��Ϊ4CH3OH��2CO��O2![]() 2(CH3O)2CO��2H2O��װ����ͼ3��ʾ��д�������ĵ缫��Ӧʽ��________________________________________
2(CH3O)2CO��2H2O��װ����ͼ3��ʾ��д�������ĵ缫��Ӧʽ��________________________________________