��Ŀ����
����Ŀ����ҵ�����в�����![]() ��NOֱ���ŷŽ��Դ������������Ⱦ�����õ绯ѧԭ������
��NOֱ���ŷŽ��Դ������������Ⱦ�����õ绯ѧԭ������![]() ��NO��ͬʱ���
��NO��ͬʱ���![]() ��
��![]() ��Ʒ�Ĺ�������ͼ����
��Ʒ�Ĺ�������ͼ����![]() Ϊ��Ԫ��
Ϊ��Ԫ��![]() ��
��

��ش��������⣮
![]() װ�â���NO����������������
װ�â���NO����������������![]() �����ӷ���ʽ ______ ��
�����ӷ���ʽ ______ ��
![]() �������
�������![]() ��
��![]() ��
��![]() ������
������![]() ��NaOH��Һ��Ӧ�����Һ�У����ǵ����ʵ�������
��NaOH��Һ��Ӧ�����Һ�У����ǵ����ʵ�������![]() ����ҺpH�Ĺ�ϵ��ͼ1��ʾ��
����ҺpH�Ĺ�ϵ��ͼ1��ʾ��
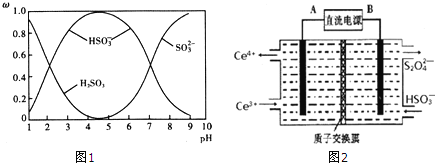
������˵����ȷ���� ______ ![]() ����
����![]() ��
��
A ![]() ʱ����Һ��
ʱ����Һ��![]()
![]()
![]()
B ��ͼ�����ݣ����Թ����![]() �ĵڶ�������ƽ�ⳣ��
�ĵڶ�������ƽ�ⳣ��![]()
C Ϊ��þ����ܴ���![]() ��Ӧ����Һ��pH������
��Ӧ����Һ��pH������![]() Ϊ��
��
D ![]() ��
��![]() ʱ����Һ�������������ͬ
ʱ����Һ�������������ͬ
����![]() ��NaOH��Һ��ȫ����
��NaOH��Һ��ȫ����![]() �����
�����![]() ����Ӧ�����ӷ���ʽΪ ______ ��
����Ӧ�����ӷ���ʽΪ ______ ��
��ȡװ�â��е�����ҺvmL����![]() �����Ը��������Һ�ζ������Ը��������ҺӦװ�� ______
�����Ը��������Һ�ζ������Ը��������ҺӦװ�� ______ ![]() ������ʽ��������ʽ��
������ʽ��������ʽ��![]() �ζ����У��жϵζ��յ�ķ����� ______ ��
�ζ����У��жϵζ��յ�ķ����� ______ ��
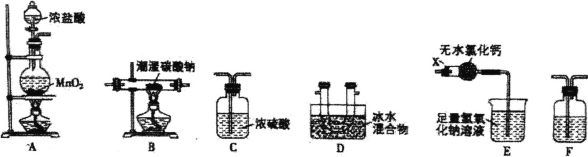
![]() װ�â������֮һ������
װ�â������֮һ������![]() ����ԭ����ͼ2��ʾ��ͼ��AΪ��Դ�� ______
����ԭ����ͼ2��ʾ��ͼ��AΪ��Դ�� ______ ![]() ����������������
����������������![]() �����Ҳ෴Ӧ���з�������Ҫ�缫��ӦʽΪ ______ ��
�����Ҳ෴Ӧ���з�������Ҫ�缫��ӦʽΪ ______ ��
![]() ��֪����װ�â�����Һ��
��֪����װ�â�����Һ��![]() ��Ũ��Ϊ
��Ũ��Ϊ![]() ��Ҫʹ
��Ҫʹ![]() ����Һ�е�
����Һ�е�![]() ��ȫת��Ϊ
��ȫת��Ϊ![]() ����������װ�â���ͨ���״���µ�
����������װ�â���ͨ���״���µ�![]() �����Ϊ ______ L��
�����Ϊ ______ L��
���𰸡�![]() BCD
BCD ![]() ��ʽ �������һ����Һ���Ϻ�ɫ�Ұ������ɫ���� ��
��ʽ �������һ����Һ���Ϻ�ɫ�Ұ������ɫ���� �� ![]() 4480
4480
��������
װ�â��ж�������������������ܺ�ǿ����������֮�䷢����Ӧ��![]() ��NO����������֮�䲻�ᷴӦ��װ�â���NO�����������£�NO��
��NO����������֮�䲻�ᷴӦ��װ�â���NO�����������£�NO��![]() ֮��ᷢ��������ԭ��Ӧ��
֮��ᷢ��������ԭ��Ӧ��![]() ��
��![]() ��װ�â��У��ڵ��۵�����
��װ�â��У��ڵ��۵�����![]() �������缫��ӦʽΪ��
�������缫��ӦʽΪ��![]() ��װ�â���ͨ�백����������
��װ�â���ͨ�백����������![]() ��
��
![]() �����Ի����£�NO��
�����Ի����£�NO��![]() ֮��ᷢ��������ԭ��Ӧ��
֮��ᷢ��������ԭ��Ӧ��
![]() ʱ����ҺΪ���ԣ���ϵ���غ������
ʱ����ҺΪ���ԣ���ϵ���غ������
B.![]() ����ͼ�����ݣ�
����ͼ�����ݣ�![]() ʱ��
ʱ��![]() ��
��
C.��Һ��pH������![]() ʱ��
ʱ��![]() Ũ�����
Ũ�����
D.��ͼ��֪��![]() ʱ��
ʱ��![]() ��
��![]() ʱ��Һ����ҺΪ����������Һ��
ʱ��Һ����ҺΪ����������Һ��
![]() �����ʵ���Ϊ1mol������
�����ʵ���Ϊ1mol������![]() ��֪��������������������ֵĶ��������ٷ�����Ӧ
��֪��������������������ֵĶ��������ٷ�����Ӧ![]() �����ݷ���ʽ���м���
�����ݷ���ʽ���м���![]() ��
��![]() �ıȣ��ݴ���д���ӷ���ʽ��
�ıȣ��ݴ���д���ӷ���ʽ��
![]() ��ʽ�ζ���ֻ��ʢ��������Һ����ʽ�ζ���ֻ��ʢ�ż�����Һ�����Ը�����ؾ���ǿ�����ԣ���������ʽ�ζ�����Ƥ�ܣ�ԭ��Һ��ɫ��
��ʽ�ζ���ֻ��ʢ��������Һ����ʽ�ζ���ֻ��ʢ�ż�����Һ�����Ը�����ؾ���ǿ�����ԣ���������ʽ�ζ�����Ƥ�ܣ�ԭ��Һ��ɫ��![]() Ϊ�Ϻ�ɫ������Һ�е�
Ϊ�Ϻ�ɫ������Һ�е�![]() ��
��![]() ��Ӧ��ȫʱ����Һ���Ϻ�ɫ�Ұ������ɫ���䣻
��Ӧ��ȫʱ����Һ���Ϻ�ɫ�Ұ������ɫ���䣻
![]() ����
����![]() Ϊ������Ӧ�������������ϣ���Ӧ����
Ϊ������Ӧ�������������ϣ���Ӧ����![]() ����ԭ��
����ԭ��![]() ���õ����ӣ�
���õ����ӣ�
![]() ��Ũ��Ϊ
��Ũ��Ϊ![]() ��Ҫʹ
��Ҫʹ![]() ����Һ�е�
����Һ�е�![]() ��ȫת��Ϊ
��ȫת��Ϊ![]() �������ı���������������V����ϵ����غ���м��㡣
�������ı���������������V����ϵ����غ���м��㡣
![]() װ�â���NO������������NO��
װ�â���NO������������NO��![]() ֮��ᷢ��������ԭ��Ӧ��
֮��ᷢ��������ԭ��Ӧ��![]() ��
��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
![]() ʱ����Һ�����ԣ�
ʱ����Һ�����ԣ�![]() ����Һ�д��ڵ���غ㣺
����Һ�д��ڵ���غ㣺![]() ������Һ��
������Һ��![]() ����A����
����A����
B.![]() ����ͼ�����ݣ�
����ͼ�����ݣ�![]() ʱ��
ʱ��![]() ����Ka�ı���ʽ��֪��
����Ka�ı���ʽ��֪��![]() �ĵڶ�������ƽ�ⳣ��
�ĵڶ�������ƽ�ⳣ��![]() ����B��ȷ��
����B��ȷ��
C.��Һ��pH������![]() ʱ��
ʱ��![]() Ũ�������Ϊ��þ����ܴ���
Ũ�������Ϊ��þ����ܴ���![]() ���ɽ���Һ��pH������
���ɽ���Һ��pH������![]() ���ң���C��ȷ��
���ң���C��ȷ��
D.��ͼ��֪��![]() ʱ��
ʱ��![]() ����ҺΪ�����ᡢ������������Һ��
����ҺΪ�����ᡢ������������Һ��![]() ʱ��Һ����ҺΪ����������Һ��������Һ�������������ͬ����D��ȷ��
ʱ��Һ����ҺΪ����������Һ��������Һ�������������ͬ����D��ȷ��
�ʴ�Ϊ��BCD��
![]() ��NaOH��Һ�к����������Ƶ����ʵ���Ϊ��
��NaOH��Һ�к����������Ƶ����ʵ���Ϊ��![]() ��
��![]() �����
�����![]() ��
��![]() ��
��
�跴Ӧ�����������Ƶ����ʵ���Ϊx�����Ķ�����������ʵ���Ϊy
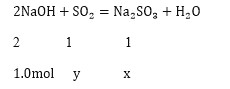
![]() ����ã�
����ã�![]() ���������������֪�������������ǹ����ģ�ʣ��Ķ�����������ʵ���Ϊ��
���������������֪�������������ǹ����ģ�ʣ��Ķ�����������ʵ���Ϊ��![]() �����Զ������������ɵ��������Ƽ�����Ӧ���������������Ƶ����ʵ���Ϊa���������������Ƶ����ʵ���Ϊb
�����Զ������������ɵ��������Ƽ�����Ӧ���������������Ƶ����ʵ���Ϊa���������������Ƶ����ʵ���Ϊb
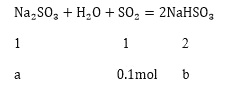
![]() ��ã�
��ã�![]() ������Һ��
������Һ��![]() ��
��![]() ��
��![]() ��
��![]() ��1����Ӧ�����ӷ���ʽΪ
��1����Ӧ�����ӷ���ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
![]() ��ʽ�ζ���ֻ��ʢ��������Һ����ʽ�ζ���ֻ��ʢ�ż�����Һ�����Ը��������Һ�����ԣ������ʢ������ʽ�ζ����У�ԭ��Һ��ɫ����
��ʽ�ζ���ֻ��ʢ��������Һ����ʽ�ζ���ֻ��ʢ�ż�����Һ�����Ը��������Һ�����ԣ������ʢ������ʽ�ζ����У�ԭ��Һ��ɫ����![]() Ϊ�Ϻ�ɫ�����Ե���Һ�е�
Ϊ�Ϻ�ɫ�����Ե���Һ�е�![]() ��
��![]() ��Ӧ��ȫʱ���������һ����Һ���Ϻ�ɫ�Ұ������ɫ���䣬�ʴ�Ϊ����ʽ���������һ����Һ���Ϻ�ɫ�Ұ������ɫ���䣻
��Ӧ��ȫʱ���������һ����Һ���Ϻ�ɫ�Ұ������ɫ���䣬�ʴ�Ϊ����ʽ���������һ����Һ���Ϻ�ɫ�Ұ������ɫ���䣻
![]() ����
����![]() Ϊ������Ӧ�������������ϣ���˵��ʱ���ɵ�
Ϊ������Ӧ�������������ϣ���˵��ʱ���ɵ�![]() �ڵ��۵����������ӵ�Դ��������Ӧ����
�ڵ��۵����������ӵ�Դ��������Ӧ����![]() ����ԭ��
����ԭ��![]() ���õ����ӣ��缫��ӦʽΪ��
���õ����ӣ��缫��ӦʽΪ��![]() ���ʴ�Ϊ������
���ʴ�Ϊ������![]() ��
��
![]() ��Ũ��Ϊ
��Ũ��Ϊ![]() ��Ҫʹ
��Ҫʹ![]() ����Һ�е�
����Һ�е�![]() ��ȫת��Ϊ
��ȫת��Ϊ![]() ����ʧȥ������Ϊ��
����ʧȥ������Ϊ��![]() �������ı���������������V�����ݵ����غ㣺
�������ı���������������V�����ݵ����غ㣺![]() �����
�����![]() ���ʴ�Ϊ��4480��
���ʴ�Ϊ��4480��

����Ŀ����2L�ܱ������ڣ�����0.100molCO�����0.080molCuO���壬800��ʱ�������·�Ӧ��2CuO��s��+CO��g��![]() Cu2O��s��+CO2��g����n��CuO����ʱ��ı仯�����
Cu2O��s��+CO2��g����n��CuO����ʱ��ı仯�����
ʱ�䣨min�� | 0 | 1 | 2 | 3 | 4 | 5 |
n��CuO����mol�� | 0.080 | 0.060 | 0.040 | 0.020 | 0.020 | 0.020 |
��1����CO��ʾǰ2min�ڵĻ�ѧ��Ӧ����=_______
��2������˷�Ӧ��800Cʱ�Ļ�ѧƽ�ⳣ��K=_______
��3������ƽ������ϵ�м���CO��CO2��0.05mol�����ʱV������_______V���棩
��4��������ԭCuO��CO������C��ˮ������Ӧ�Ƶá�
��֪��C��s����O 2��g��= CO2��g�� H=-393.5kJ/mol
2CO(g)+O2(g)=2CO2(g) H=-566kJ/mol
2H2(g)+O2(g)=2H2O(g) H=-571.6kJ/mol
��C��s����H2O��g��![]() CO��g����H2��g�� H= __________��
CO��g����H2��g�� H= __________��
����Ŀ���±��ṩ���Լ�������ͼ��ʾװ�ã����Ʊ���Ӧ�������
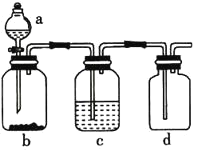
ѡ�� | a���Լ� | b���Լ� | c���Լ� | ʵ��Ŀ�� |
A. | Ũ���� | �������� | ����ʳ��ˮ | �Ʊ����ռ����� |
B. | ϡ���� | ʯ��ʯ | Ũ���� | �Ʊ����ռ�������̼ |
C. | ϡ���� | ��п | Ũ���� | �Ʊ����ռ����� |
D. | ϡ���� | ͭ�� | Ũ���� | �Ʊ����ռ�һ������ |
A.AB.BC.CD.D
����Ŀ����֪��4CO(g)+2NO2(g)4CO2(g)+N2(g) ��H����1200 kJ��mol1����2 L�����ܱ������У������±��мס������ַ�ʽ����Ͷ�ϣ�����һ��ʱ���ﵽƽ��״̬����ü���CO��ת����Ϊ50%������˵������ȷ����
�� | �� |
0.2 mol NO2 | 0.1 mol NO2 |
0.4 mol CO | 0.2 mol CO |
A.���ȷ�Ӧ��һ�����Է�����
B.���¶��£���Ӧ��ƽ�ⳣ��Ϊ5
C.��ƽ��ʱ��NO2��Ũ�ȣ��ף���
D.��ƽ��ʱ��N2������������ף���
����Ŀ����������ﮣ�LiFePO4�����������Դ�����Ķ������֮һ���Ͼɵ������Ƭ����������ﮡ�̿�ں������ȣ����������ã��乤���������£�

��֪��̼�����ˮ�е��ܽ�ȣ�0��ʱΪ1.54g��90��ʱΪ0.85g��100��ʱΪ0.71g��
��1������������������Ҫ����______�ι��˲�����
��2����������������Ӧ�����ӷ���ʽΪ__________������HNO3����H2O2����֮����_____��
��3������֪Ksp[Fe(OH)3]=2.6��10-39�������£���Fe(OH)3����Һ�У�����Һ��pH=3.0ʱ��Fe3+��Ũ��Ϊ________mol/L��
��ʵ�����������У�������pH�����ɳ���ʱ����ҺpH�����Ԫ�صij����ٷ���(��)�Ĺ�ϵ���±���
pH | 3.5 | 5.0 | 6.5 | 8.0 | 10.0 | 12.0 |
��(Fe)/% | 66.5 | 79.2 | 88.5 | 97.2 | 97.4 | 98.1 |
��(Li)/% | 0.9 | 1.3 | 1.9 | 2.4 | 4.5 | 8.0 |
����ѵij���pH=________��
��4�������" ʱ���¶�Ӧѡ�����ţ�______Ϊ�ˣ�����___ϴ�ӣ����ˮ" ����ˮ"����
a��90�� b��60 �� c��30 �� d��0 ��
��5����������﮵���ڹ���ʱ����������LiFePO4��FePO4��ת�����õ�طŵ�ʱ�����ĵ缫��ӦʽΪ________��
��6����ҵ�Ͽ�����FePO4��Li2CO3��H2C2O4��ԭ�ϸ��±����Ʊ� LiFePO4���÷�Ӧ�Ļ�ѧ����ʽΪ________