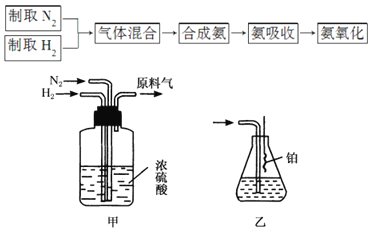
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΚœάμ¥Πάμ»ΦΤχ÷–ΒΡH2SΘ§≤ΜΫωΩ…Φθ…ΌΕ‘¥σΤχΒΡΈέ»ΨΘ§ΜΙΩ…Ϋχ––Ή ‘¥Μ·άϊ”ΟΓΘΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©H2SΚΆSΒΡ»Φ…’»»»γ±μΥυ ΨΘ§
Έο÷ | »Φ…’»»/ΓςHΘ®kJmol-1Θ© |
H2S | -a |
S | -b |
«κ–¥≥ω≥ΘΈ¬œ¬H2S”κSO2Ζ¥”ΠΒΡ»»Μ·―ßΖΫ≥Χ Ϋ______ΓΘ
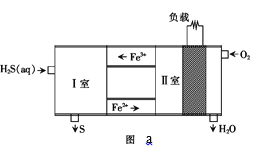
Θ®2Θ©Ω…“‘Α―H2S…ηΦΤΈΣ“Μ÷÷»ΦΝœΒγ≥ΊΘ§‘≠άμ»γΆΦaΥυ ΨΘΚΤδ÷–Θ§Fe2+‘ΎΒγ≥ΊΙΛΉς÷–ΒΡΉς”Ο «______ΘΜ«κ–¥≥ω I “ΖΔ…ζΒΡάκΉ”ΖΫ≥Χ Ϋ______ΓΘ
Θ®3Θ©ΈΣΧΫΨΩH2SΒΡ÷±Ϋ”»»Ϋβ2H2SΘ®gΘ©=2H2Θ®g Θ©+S2Θ®gΘ©ΓΘ‘Ύ“ΜΧεΜΐΈΣ2LΒΡΟή±’»ίΤς÷–≥δ»κ2 mol H2S”κ1 molAr Θ®ΤπΒΫœΓ ΆΉς”ΟΘ©Θ§Ϋχ–– Β―ιΓΘ
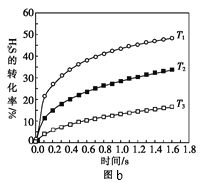
ΔΌΡ≥Ά§―ß≤β≥ω≤ΜΆ§Έ¬Ε»œ¬H2SΒΡΈο÷ ΒΡΝΩ”κΖ¥”Π ±ΦδΒΡΆΦœσΘ§ΆΦb «ΫΊ»ΓΗΟΆΦœσΒΡ÷–ΦδΡ≥≤ΩΖ÷ΓΘ«κΦΤΥψT2Έ¬Ε»œ¬Θ§0-l0sΡΎΘ§H2SΒΡΖ¥”ΠΥΌ¬ v=______moL-1s-1ΘΜ
ΔΎΆΦb÷–Θ§T1ΓΔT2ΓΔT3»ΐΗωΈ¬Ε»Θ§ΉνΗΏΒΡ «______ΘΜ±»ΫœAΒψ”κBΒψΒΡΡφΖ¥”ΠΥΌ¬ ΒΡ¥σ–ΓΘ§vAΘ®ΡφΘ©______vBΘ®ΡφΘ©Θ®ΧνΓΑΘΨΓ±ΓΔΓΑΘΦΓ±ΜρΓΑ=Γ±Θ©ΘΜ
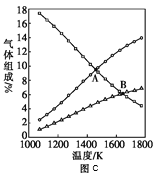
ΔέΆΦc «±μ ΨΖ¥”Π2H2SΘ®gΘ©=2H2Θ®gΘ©+S2Θ®gΘ©ΤΫΚβ ±Θ§ΉΑ÷ΟΡΎΗςΉιΖ÷ΤχΧεΈο÷ ΒΡΝΩΖ÷ ΐ=”κΈ¬Ε»ΒΡΙΊœΒΘ®Τδ÷–ArΤχΧε±δΜ·«ζœΏΈ¥Μ≠≥ωΘ©ΓΘ«κΦΤΥψΘΚCΒψΒΡΤΫΚβ≥Θ ΐK=______ΘΜDΒψΤΫΚβ ±Θ§H2SΒΡΉΣΜ·¬ =______ΓΘ
ΓΨ¥πΑΗΓΩ2H2SΘ®gΘ©+SO2Θ®gΘ©=3SΘ®sΘ©+2H2OΘ®lΘ©ΓςH=Θ®-2a+3bΘ©kJ/mol ¥ΏΜ·ΦΝ 2Fe3++H2S=2Fe2++2H++SΓΐ 0.04 T3 ΘΨ 0.25 66.7%
ΓΨΫβΈωΓΩ
Δ≈H2SΒΡ»Φ…’»»Μ·―ßΖΫ≥Χ ΫΘΚH2S(g) +![]() O2(g)=SO2(g)+H2O(l) ΓςH= Θ≠a kJΓΛmol-1Θ§SΒΡ»Φ…’»»Μ·―ßΖΫ≥Χ ΫΘΚS(s) +O2(g) =SO2(g) ΓςH=Θ≠bkJΓΛmol-1Θ§ΗυΨί»»Μ·―ßΖΫ≥Χ ΫΆΤΒΦΥυ«σΖ¥”ΠΘ§‘ΌΗυΨίΗ«ΥΙΕ®¬…«σΓςHΓΘ
O2(g)=SO2(g)+H2O(l) ΓςH= Θ≠a kJΓΛmol-1Θ§SΒΡ»Φ…’»»Μ·―ßΖΫ≥Χ ΫΘΚS(s) +O2(g) =SO2(g) ΓςH=Θ≠bkJΓΛmol-1Θ§ΗυΨί»»Μ·―ßΖΫ≥Χ ΫΆΤΒΦΥυ«σΖ¥”ΠΘ§‘ΌΗυΨίΗ«ΥΙΕ®¬…«σΓςHΓΘ
ΔΤFe2+‘ΎΒγ≥Ί÷–≥ œ÷―≠ΜΖΘ§ΗυΨί¥ΏΜ·ΦΝΧΊΒψ≈–ΕœΘ§I “Fe3+ΫΪH2S―θΜ·ΈΣSΘ§Ψί¥Υ ι–¥ΖΫ≥Χ ΫΓΘ
Δ«ΔΌΖ¥”ΠΥΌ¬ ΈΣΒΞΈΜ ±ΦδΡΎ≈®Ε»ΒΡ±δΜ·ΝΩΘ§ΗυΨίΆΦœσ Θ”ύ1.2mol H2SΘ§ΫαΚœΤπ ΦΝΩΦ¥Ω…ΦΤΥψH2SΒΡΖ¥”ΠΥΌ¬ ΘΜΔΎΗυΨίΈϋ»»Ζ¥”ΠΘ§Έ¬Ε»‘ΫΗΏΘ§ΉΣΜ·¬ ‘ΫΗΏΘΜΫαΚœΈ¬Ε»Ε‘Ζ¥”ΠΥΌ¬ ΒΡ”ΑœλΘ§Έ¬Ε»‘ΫΗΏΘ§Ζ¥”ΠΥΌ¬ ‘ΫΗΏΘΜΔέ“―÷ΣH2SΤπ ΦΝΩΘ§Ω……ηΤδ±δΜ·ΝΩΈΣxmolΘ§Ν–≥ω»ΐΕΈ ΫΘ§‘ΎΗυΨί≤ΜΆ§ΧθΦΰœ¬≤ΜΆ§Έο÷ Έο÷ ΒΡΝΩΒΡΙΊœΒΫβ¥πxΘ§ΉνΚσ«σΫβCΒψΒΡΤΫΚβ≥Θ ΐKΘ§H2SΒΡΉΣΜ·¬ ΓΘ
Δ≈H2SΒΡ»Φ…’»»Μ·―ßΖΫ≥Χ ΫΘΚH2S(g) +![]() O2(g)=SO2(g)+H2O(l) ΓςH=Θ≠a kJΓΛmol-1Θ§SΒΡ»Φ…’»»Μ·―ßΖΫ≥Χ ΫΘΚS(s) +O2(g) =SO2(g) ΓςH=Θ≠bkJΓΛmol-1Θ§ΗυΨίΗ«ΥΙΕ®¬…Θ§H2S”κSO2Ζ¥”ΠΒΡ»»Μ·―ßΖΫ≥Χ ΫΈΣΘΚ2H2S(g) +SO2(g) =3S(s) +2H2O(l)ΓςH=(Θ≠2a+3b) kJΓΛmol-1Θ§Ι ¥πΑΗΈΣΘΚ2H2S(g) +SO2(g) =3S(s) +2H2O(l)ΓςH=(Θ≠2a+3b) kJΓΛmol-1ΓΘ
O2(g)=SO2(g)+H2O(l) ΓςH=Θ≠a kJΓΛmol-1Θ§SΒΡ»Φ…’»»Μ·―ßΖΫ≥Χ ΫΘΚS(s) +O2(g) =SO2(g) ΓςH=Θ≠bkJΓΛmol-1Θ§ΗυΨίΗ«ΥΙΕ®¬…Θ§H2S”κSO2Ζ¥”ΠΒΡ»»Μ·―ßΖΫ≥Χ ΫΈΣΘΚ2H2S(g) +SO2(g) =3S(s) +2H2O(l)ΓςH=(Θ≠2a+3b) kJΓΛmol-1Θ§Ι ¥πΑΗΈΣΘΚ2H2S(g) +SO2(g) =3S(s) +2H2O(l)ΓςH=(Θ≠2a+3b) kJΓΛmol-1ΓΘ
ΔΤFe2+‘ΎΒγ≥ΊΙΛΉς÷–Ιΐ≥Χ÷–―≠ΜΖάϊ”ΟΘ§–‘÷ ÷ ΝΩΟΜ”–ΗΡ±δΘ§Υυ“‘Fe2+ΈΣ¥ΏΜ·ΦΝΘ§I “ΖΔ…ζΒΡΖ¥”ΠΈΣFe3+―θΜ·H2SΘ§άκΉ”ΖΫ≥Χ ΫΈΣΘΚ2Fe3++H2S=2Fe2++2H++SΓΐΘ§Ι ¥πΑΗΈΣΘΚ2Fe3++H2S= 2Fe2++2H++SΓΐΓΘ
Δ«ΔΌΤπ Φ ±≥δ»κ2molH2SΘ§ΗυΨίΆΦœσΩ…÷Σ10s ± Θ”ύ1.2molH2SΘ§‘ρ![]() Θ§Ι ¥πΑΗΈΣΘΚ0.04ΓΘ
Θ§Ι ¥πΑΗΈΣΘΚ0.04ΓΘ
ΔΎΗυΨίΖ÷ΫβΖ¥”Π“ΜΑψΈΣΈϋ»»Ζ¥”ΠΘ§Έ¬Ε»‘ΫΗΏΘ§ΉΣΜ·¬ ‘ΫΗΏΘ§‘ρT3Έ¬Ε»ΉνΗΏΘ§AΒψ”κBΒψΥυΕ‘”ΠΒΡΗςΈο÷ ≈®Ε»ΨυœύΆ§Θ§ΒΪAΒψΕ‘”ΠΒΡT3ΉνΗΏΘ§Ζ¥”ΠΥΌ¬ œύΕ‘ΫœΗΏΘ§Υυ“‘vA(Ρφ)ΘΨvB(Ρφ)Θ§Ι ¥πΑΗΈΣΘΚT3ΘΜΘΨΓΘ
Δέ”…”ΎΤπ Φ ±2 molH2SΘ§…ηH2S±δΜ·ΝΩΈΣxmolΘ§Ν–»ΐΕΈ ΫΘΚ
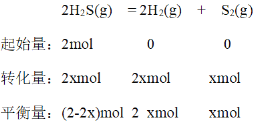
CΒψ ±ΘΚΤΫΚβ ±H2S”κH2ΒΡΈο÷ ΒΡΝΩΖ÷ ΐœύΆ§Θ§‘ρΤΫΚβ ±H2S”κH2ΒΡΈο÷ ΒΡΝΩœύΆ§Θ§‘ρ2-2x=2xΘ§x=0.5Θ§‘ρΤΫΚβ ±c(H2S) = 0.5molL-1Θ§c(H2)=0.5 molL-1Θ§c(S2)=0.25 molL-1Θ§ΤΫΚβ≥Θ ΐ![]() ΘΜDΒψ ±ΘΚΤΫΚβ ±S2”κH2SΒΡΈο÷ ΒΡΝΩΖ÷ ΐœύΆ§Θ§‘ρΤΫΚβ ±S2”κH2SΒΡΈο÷ ΒΡΝΩœύΆ§Θ§‘ρ2-2x=xΘ§ΫβΒΟx=
ΘΜDΒψ ±ΘΚΤΫΚβ ±S2”κH2SΒΡΈο÷ ΒΡΝΩΖ÷ ΐœύΆ§Θ§‘ρΤΫΚβ ±S2”κH2SΒΡΈο÷ ΒΡΝΩœύΆ§Θ§‘ρ2-2x=xΘ§ΫβΒΟx= ![]() Θ§H2SΒΡΉΣΜ·¬
Θ§H2SΒΡΉΣΜ·¬ 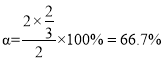 ΘΜΙ ¥πΑΗΈΣΘΚ0.25ΘΜ66.7%ΓΘ
ΘΜΙ ¥πΑΗΈΣΘΚ0.25ΘΜ66.7%ΓΘ

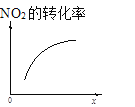
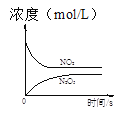
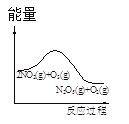
ΓΨΧβΡΩΓΩ≥τ―θ «άμœκΒΡ―ΧΤχΆ―œθ ‘ΦΝΘ§ΤδΆ―œθΖ¥”ΠΈΣΘΚ2NO2(g)+O3(g)N2O5(g)+O2(g)Θ§»τΖ¥”Π‘ΎΚψ»ίΟή±’»ίΤς÷–Ϋχ––Θ§œ¬Ν–”…ΗΟΖ¥”ΠœύΙΊΆΦœώΉς≥ωΒΡ≈–Εœ’ΐ»ΖΒΡ «
A | B | C | D |
|
|
|
|
t1 ±ΫωΦ”»κ¥ΏΜ·ΦΝΘ§ΤΫΚβœρ’ΐΖΫœρ“ΤΕ· | ¥οΤΫΚβ ±Θ§ΫωΗΡ±δxΘ§‘ρxΈΣc(O2) | ¥”Ζ¥”ΠΩΣ Φ¥οΤΫΚβΤχΧε≈®Ε»±δΜ· | …ΐΗΏΈ¬Ε»Θ§ΤΫΚβ≥Θ ΐΦθ–Γ |
A.AB.BC.CD.D
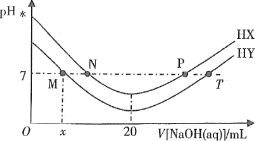
ΓΨΧβΡΩΓΩ‘Ύ“ΜΕ®Έ¬Ε»œ¬Θ§ΫΪΤχΧεX”κΤχΧεYΗς0.16mol≥δ»κ10LΚψ»ίΟή±’»ίΤς÷–Θ§ΖΔ…ζΖ¥”ΠΘΚXΘ®gΘ©+YΘ®gΘ©2ZΘ®gΘ©ΓςHΘΦ0Θ§“ΜΕΈ ±ΦδΚσ¥οΒΫΤΫΚβΘ°Ζ¥”ΠΙΐ≥Χ÷–≤βΕ®ΒΡ ΐΨί»γ±μΘΚœ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «Θ®ΓΓΓΓΘ©
t®Mmin | 2 | 4 | 7 | 9 |
nΘ®YΘ©®Mmol | 0.12 | 0.11 | 0.10 | 0.10 |
A. Ζ¥”Π«Α4minΒΡΤΫΨυΖ¥”ΠΥΌ¬ Π‘Θ®ZΘ©=0.0125molL-1min-1
B. ΤδΥϊΧθΦΰ≤Μ±δΘ§ΫΒΒΆΈ¬Ε»Θ§Ζ¥”Π¥οΒΫ–¬ΤΫΚβ«ΑΠ‘Θ®ΡφΘ©ΘΨΠ‘Θ®’ΐΘ©
C. ΤδΥϊΧθΦΰ≤Μ±δΘ§‘Ό≥δ»κ0.2molZΘ§¥οΤΫΚβ ±XΒΡΧεΜΐΖ÷ ΐ‘ω¥σ
D. ΗΟΈ¬Ε»œ¬¥ΥΖ¥”ΠΒΡΤΫΚβ≥Θ ΐK=1.44
ΓΨΧβΡΩΓΩ‘Ύ100Γφ ±Θ§ΫΪ0.40 molΕΰ―θΜ·ΒΣΤχΧε≥δ»κ“ΜΗω2 L≥ιΩ’ΒΡΟή±’»ίΤς÷–Θ§ΖΔ…ζΖ¥”ΠΘΚ2NO2 N2O4ΓΘΟΩΗτ“ΜΕΈ ±ΦδΨΆΕ‘ΗΟ»ίΤςΡΎΒΡΈο÷ Ϋχ––Ζ÷ΈωΘ§ΒΟΒΫœ¬±μ ΐΨίΘΚ
±Φδ/s | 0 | 20 | 40 | 60 | 80 |
n(NO2)/mol | 0.40 | n1 | 0.26 | n3 | n4 |
n(N2O4)/mol | 0.00 | 0.05 | n2 | 0.08 | 0.08 |
Θ®1Θ©‘Ύ…œ ωΧθΦΰœ¬Θ§¥”Ζ¥”ΠΩΣ Φ÷Ν20 s ±Θ§”ΟNO2±μ ΨΒΡΤΫΨυΖ¥”ΠΥΌ¬ ΈΣ__________molΓΛL1ΓΛs1ΓΘ
Θ®2Θ©n3________(ΧνΓΑ>Γ±ΓΑ<Γ±ΜρΓΑ=Γ±)n4ΘΜΗΟΖ¥”ΠΒΡΤΫΚβ≥Θ ΐKΒΡ ΐ÷ΒΈΣ___________(ΨΪ»ΖΒΫ0.1)ΓΘ
Θ®3Θ©»τ‘ΎœύΆ§ΧθΦΰœ¬Ήν≥θœρΗΟ»ίΤς÷–≥δ»κN2O4Θ§“Σ¥οΒΫ…œ ωΤΫΚβΉ¥Χ§Θ§N2O4ΒΡΤπ Φ≈®Ε» «______molΓΛL1ΓΘ
Θ®4Θ©…œ ωΘ®3Θ©¥οΒΫΤΫΚβΚσN2O4ΒΡΉΣΜ·¬ ΈΣ______________Θ§ΜλΚœΤχΧεΒΡΤΫΨυΡΠΕϊ÷ ΝΩΈΣ______________ΓΘ
Θ®5Θ©¥οΒΫΤΫΚβΚσΘ§»γΙϊ…ΐΗΏΈ¬Ε»Θ§ΤχΧε―’…ΪΜα±δ…νΘ§‘ρ…ΐΗΏΈ¬Ε»ΚσΘ§Ζ¥”Π2NO2N2O4ΒΡΤΫΚβ≥Θ ΐΫΪ_______________(ΧνΓΑ‘ω¥σΓ±ΓΑΦθ–ΓΓ±ΜρΓΑ≤Μ±δΓ±)ΓΘ
Θ®6Θ©¥οΒΫΤΫΚβΚσΘ§»γΙϊœρΗΟΟή±’»ίΤς÷–‘Ό≥δ»κ0.32 mol HeΘ§≤ΔΑ―»ίΤςΧεΜΐά©¥σΈΣ4 LΘ§‘ρΤΫΚβΫΪ______________Θ®ΧνΓΑœρΉσ“ΤΕ·Γ±ΓΑœρ”““ΤΕ·Γ±ΜρΓΑ≤Μ“ΤΕ·Γ±Θ©ΓΘ