��Ŀ����
15��ij��������Һ�У����ܴ����������������е������֣�H+��NH4+��K+��Mg2+��Cu2+��Br-��AlO2-��Cl-��SO42-��CO32-���ֽ�������ʵ�飺�����Թ�ȡ������Һ����μ���ϡ��������������Һ�Ȼ��Ǻ��ֱ���壬����ɫ����ų�������Һ��Ϊ3�ݣ�
���ڵ�1����Һ����μ���NaOH��Һ����������Һ�Ȼ��Ǻ��ֱ���壮���ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�δ����������
���ڵ�2����Һ�м������Ƶ���ˮ��CCl4�����ã��²���Һ�ԳȺ�ɫ��
�������ƶ���ȷ���ǣ�������
| A�� | ��Һ��һ����K+��Br-��CO32-��AlO2- | |
| B�� | ��Һ��һ��û��Mg2+��Cu2+��Cl-��NH4+ | |
| C�� | ����ȷ����Һ���Ƿ���K+��SO42-��Cl- | |
| D�� | ����3����Һ�еμ�BaCl2��ȷ���Ƿ���SO42- |
���� �����Թ�ȡ������Һ����μ���ϡ��������������Һ�Ȼ��Ǻ��ֱ���壬����ɫ����ų�����֪��ɫ����Ϊ������̼��һ����CO32-����Һ�Ȼ��Ǻ��ֱ�����֪һ����AlO2-�������ӹ����֪��һ������H+��Mg2+��Cu2+��
����һ����Һ����μ���NaOH��Һ����������Һ�Ȼ��Ǻ��ֱ���壬���ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�δ������������NH4+��
������һ����Һ�м������Ƶ���ˮ��CCl4�����ã��²���Һ�ԳȺ�ɫ����һ����Br-����ϵ���غ㼰���ӹ�����
��� �⣺�����Թ�ȡ������Һ����μ���ϡ��������������Һ�Ȼ��Ǻ��ֱ���壬����ɫ����ų�����֪��ɫ����Ϊ������̼��һ����CO32-����Һ�Ȼ��Ǻ��ֱ�����֪һ����AlO2-�������ӹ����֪��һ������H+��Mg2+��Cu2+��
����һ����Һ����μ���NaOH��Һ����������Һ�Ȼ��Ǻ��ֱ���壬���ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�δ������������NH4+��
������һ����Һ�м������Ƶ���ˮ��CCl4�����ã��²���Һ�ԳȺ�ɫ����һ����Br-���ɵ���غ��֪һ������������ΪK+������ȷ���Ƿ�Cl-��SO42-��
A��������������֪��Һ��һ����K+��Br-��CO32-��AlO2-����A��ȷ��
B������ȷ���Ƿ�Cl-����B����
C����Һ��һ����K+����C����
D�����ڵ�3����Һ�����Ѿ�����HCl��Ӧ����CO32-�����ٵμ�BaCl2�����ɵij�����ΪBaSO4������ȷ��SO42-�Ĵ��ڣ���D��ȷ��
��ѡAD��
���� ���⿼�����ӵĹ��棬Ϊ��Ƶ���㣬��Ϣ���ϴ���Ҫѧ�������Ķ���Ϣ���������ӵķ�Ӧ����������۵Ĺ�ϵΪ���Ĺؼ������ط������ƶ������Ŀ��飬��Ŀ�ѶȲ���

| A�� | �����ȴ����������� | |
| B�� | �����ĸ�ʴ������������Ĥ�����ܱ����ڲ���� | |
| C�� | ��������������ʴʱ��������ӦʽΪ��O2+2H2O+4e��4OH- | |
| D�� | Ϊ�������¸ֹܲ��ܸ�ʴ����ʹ����ֱ����Դ�������� |
| A�� | �ȴ�������ˮʱ�����ļ���ɱ��Ч�����ڶ����� | |
| B�� | ���û�ѧ��Ӧ��ʵ��12C��14C��ת�� | |
| C�� | ��ѪҺ���������˽�������� | |
| D�� | ���������������ġ�PM2.5���Ƕ�һ���·��ӵ����� |
| A�� | NH3$��_{����}^{O_{2}}$NO$\stackrel{O_{2}•H_{2}O}{��}$HNO3 | |
| B�� | Ũ����ˮ$\stackrel{CI_{2}}{��}$Br2$\stackrel{�����ȿ���}{��}$Br2���ֲ�Ʒ��$\stackrel{����}{��}$Br2 | |
| C�� | MnO2$��_{����}^{ŨHCI}$Cl2$\stackrel{Ca��OH��_{2}}{��}$Ư�� | |
| D�� | ʯ��$\stackrel{�ѽ�}{��}$��ϩ$��_{����}^{����}$����ϩ |
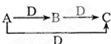 ����A��D�����ǣ�������
����A��D�����ǣ���������C��O2 ��Na��O2 ��NaOH��CO2 ��S��O2 ��Fe��Cl2��
| A�� | �٢ڢ� | B�� | �ܢ� | C�� | �٢ڢ� | D�� | �٢ڢۢ� |
| A�� | A+���Ӱ뾶С��B+���Ӱ뾶 | |
| B�� | B������������Ԫ���н�������ǿ�� | |
| C�� | D��������Ϊ�������������ˮ���Ƶ�D�ĺ����� | |
| D�� | D��E��ɵĻ������У���ԭ���������ﵽ8���ӽṹ |
| A�� | Ư����Һ�ٿ�����ʧЧ��ClO-+CO2+H2O�THClO+HCO${\;}_{3}^{-}$ | |
| B�� | ��Ũ�����뷴Ӧ��ȡ��������MnO2+2H++2Cl-$\frac{\underline{\;\;��\;\;}}{\;}$Mn2++Cl2��+2H2O | |
| C�� | ��NaAlO2��Һ��ͨ�������CO2��Al��OH��3 AlO2 -+CO2+2H2O=Al��OH��3��+HCO3- | |
| D�� | ��ǿ����Һ�У�����������Fe��OH��3��Ӧ����Na2FeO4��3ClO-+2Fe��OH��3�T2FeO${\;}_{4}^{2-}$+3Cl-+H2O+4H+ |
| A�� | ʯ���ǻ���������Ʒ�����Ǵ����� | |
| B�� | ��ɫ��Ӧ��������ɫ���֣������ǻ�ѧ��Ӧ | |
| C�� | �Ӻ�ˮ����ȡ���ʶ�����ͨ����ѧ��Ӧ����ʵ�� | |
| D�� | ������ʯȼ�����������������һ����Ҫ���� |