��Ŀ����
����Ŀ����ͼ�л�����B����ʹ��ˮ��ɫ�������ܽ�̼��ƣ�D��E��Ϊ�߷��ӻ����������ͼ��գ�

��1��д��������������������ͭ��Һ��Ӧ�Ļ�ѧ����ʽ��_____��
��2��A�еĹ�������_____(д�ṹ��ʽ)��
��3��E����Ȼ���пɽ��⣬�Ի�����������˵����ȷ����___��
A����A����E�ķ�Ӧ���ڼӾ۷�Ӧ
B��E����Է�������Ϊ72
C��E����һ���Կ�ͺ������ڼ��ٰ�ɫ��Ⱦ
��4���ڷ�Ӧ�٣����У�����ȡ����Ӧ����_____��д��D��F�Ľṹ��ʽ��D_______��F_______��
��5��A�������������ÿ��Ƶ�һ�ֳ��õ����岹�Ƽ���������ֲ��Ƽ��Ĵ������и�Ԫ�ص���������Ϊ13.0������ᾧˮ�ĺ���Ϊ_____��
���𰸡�CH2OH��CHOH��4CHO+NaOH+2Cu��OH��2![]() CH2OH��CHOH��4COONa+Cu2O��+3H2O ��OH����COOH C �ڢ�
CH2OH��CHOH��4COONa+Cu2O��+3H2O ��OH����COOH C �ڢ� ![]()
 29.2%
29.2%
��������
��������������������������ᣬAΪ![]() ������ͨ�����۷�Ӧ���ɸ߷��ӻ�����E��������B����ʹ��ˮ��ɫ�������ܽ�̼��ƣ�˵��BΪCH2=CHCOOH��A��BΪ��ȥ��Ӧ��B��״�����������Ӧ���ɵ�CΪCH2=CHCOOCH3��C�����Ӿ۷�Ӧ���ɸ߷��ӻ�����D������ͨ�����Ӽ��������ɻ�״������F��
������ͨ�����۷�Ӧ���ɸ߷��ӻ�����E��������B����ʹ��ˮ��ɫ�������ܽ�̼��ƣ�˵��BΪCH2=CHCOOH��A��BΪ��ȥ��Ӧ��B��״�����������Ӧ���ɵ�CΪCH2=CHCOOCH3��C�����Ӿ۷�Ӧ���ɸ߷��ӻ�����D������ͨ�����Ӽ��������ɻ�״������F��
��1���������к���ȩ�����ܹ�������������ͭ����Ϊ�Ȼ�����ѧ����ʽΪCH2OH��CHOH��4CHO+NaOH+2Cu��OH��2![]() CH2OH��CHOH��4COONa+Cu2O��+3H2O��
CH2OH��CHOH��4COONa+Cu2O��+3H2O��
��2����������������������������ᣬAΪ![]() �������ǻ����Ȼ����ṹ��ʽΪ��OH����COOH��
�������ǻ����Ȼ����ṹ��ʽΪ��OH����COOH��
��3������ͨ�����۷�Ӧ���ɸ߷��ӻ�����E��E����Ȼ���пɽ��⣬�Ի�������E�Ǹ߷��ӻ������Է�������һ������72������C��ȷ��AB����ѡ��C��
��4����Ӧ��Ϊ��ȥ��Ӧ����������Ӧ��ȡ����Ӧ����Ϊ�Ӿ۷�Ӧ����ѧ����ʽΪnCH2=CHCOOCH3![]()
![]() ����Ϊ���۷�Ӧ����Ϊ������Ӧ��ȡ����Ӧ����Ӧ����ʽΪ2
����Ϊ���۷�Ӧ����Ϊ������Ӧ��ȡ����Ӧ����Ӧ����ʽΪ2![]()
![]()
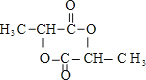 +2H2O������ȡ����Ӧ���Ǣڢݣ�D��F�Ľṹ��ʽ�ֱ���
+2H2O������ȡ����Ӧ���Ǣڢݣ�D��F�Ľṹ��ʽ�ֱ���![]() ��
��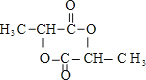 ��
��
��5�����������ˮ�ĺ���Ϊx���������ʽΪCaC6H10O6��xH2O����Ԫ�ص���������Ϊ13.0%������40/��40+12��6+1��10+16��6+18x����100%=13%��x=5����ᾧˮ�ĺ���Ϊ5��18/(40+12��6+1��10+16��6+5��18)��100%=29.2%��

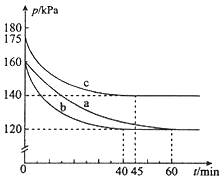
����Ŀ����ѧ��һ����ʵ��Ϊ������ѧ�ƣ�ʵ��̽���ܼ���ѧ��ѧϰ��ѧ����Ȥ��ij��ѧ��ȤС�������ͼʵ��װ�ã��г��豸���ԣ��Ʊ�������̽����������±��Ԫ�ص����ʡ��ش��������⣺

(1)����a��������______________��
(2)Aװ���з����Ļ�ѧ��Ӧ����ʽΪ_________________________________������Ư�ۻ���KClO3����Ӧ��ÿ����21.3g Cl2ʱת�Ƶĵ�����ĿΪ____NA��
(3)װ��B�����ڼ��ʵ�������C���Ƿ��������C�������˶�������B�пɹ۲쵽__________��
(4)װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ���ʱC�Т������������οɷ���____����ѡ��a��b��c����
ѡ�� | �� | �� | �� |
a | �������ɫ���� | Ũ���� | ʪ�����ɫ���� |
b | ʪ�����ɫ���� | ��ˮ�Ȼ��� | �������ɫ���� |
c | ʪ�����ɫ���� | ��ʯ�� | �������ɫ���� |
(5)���װ��D��E��Ŀ���DZȽ��ȡ��塢��ķǽ����ԡ�����D�л���ͨ����������ʱ���ɹ۲쵽��ɫ��Һ��Ϊ����ɫ��˵���ȵķǽ����Դ����壬��������D��������Һ����E�У���E���۲쵽��������_______________________________��������_____����������������������˵����ķǽ�����ǿ�ڵ⣬ԭ����_____________________��