��Ŀ����
����Ŀ����ѧ��һ����ʵ��Ϊ������ѧ�ƣ�ʵ��̽���ܼ���ѧ��ѧϰ��ѧ����Ȥ��ij��ѧ��ȤС�������ͼʵ��װ�ã��г��豸���ԣ��Ʊ�������̽����������±��Ԫ�ص����ʡ��ش��������⣺
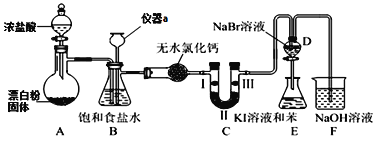
(1)����a��������______________��
(2)Aװ���з����Ļ�ѧ��Ӧ����ʽΪ_________________________________������Ư�ۻ���KClO3����Ӧ��ÿ����21.3g Cl2ʱת�Ƶĵ�����ĿΪ____NA��
(3)װ��B�����ڼ��ʵ�������C���Ƿ��������C�������˶�������B�пɹ۲쵽__________��
(4)װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ���ʱC�Т������������οɷ���____����ѡ��a��b��c����
ѡ�� | �� | �� | �� |
a | �������ɫ���� | Ũ���� | ʪ�����ɫ���� |
b | ʪ�����ɫ���� | ��ˮ�Ȼ��� | �������ɫ���� |
c | ʪ�����ɫ���� | ��ʯ�� | �������ɫ���� |
(5)���װ��D��E��Ŀ���DZȽ��ȡ��塢��ķǽ����ԡ�����D�л���ͨ����������ʱ���ɹ۲쵽��ɫ��Һ��Ϊ����ɫ��˵���ȵķǽ����Դ����壬��������D��������Һ����E�У���E���۲쵽��������_______________________________��������_____����������������������˵����ķǽ�����ǿ�ڵ⣬ԭ����_____________________��
���𰸡�����©�� Ca(ClO)2+4HCl(Ũ)�TCaCl2+2Cl2��+2H2O 0.5 Һ����볤��©���У���ƿ��Һ���½�������©����Һ���������γ�һ��Һ�� b ��Һ��Ϊ���㣬�ϲ�(����)Ϊ�Ϻ�ɫ ���� ������Cl2Ҳ�ɽ�I-����ΪI2
��������
(1)����ͼʾװ���������ṹʶ�����ƣ�
(2)������ƾ���ǿ�����ԣ��ܹ��������ᣬ���ݻ��ϼ��������������ƽ����ʽ������ΪKClO3��������Ҳ��ǿ�����ԣ���������HClΪCl2�����ݷ���ʽ���ʱ仯�����ת�ƹ�ϵ������
(3)װ��B���ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������������ʱB�е�ѹǿ����B�г���©����Һ���������γ�ˮ����
(4)Ϊ����֤�����Ƿ����Ư���ԣ�Ҫ��֤����������Ư���ԣ�ʪ�����ɫ�����У�������ˮ��Ӧ���ɴ��������Ư���ԣ�
(5)����������ǿ���壬�����ܹ��û�������������ǿ�ڵ⣬�ܹ��û�����������������ǿ�ڵ⣬�ܹ��û��⣬��ϵ����л������ܽ��Լ���ɫ�����
(1)����ͼʾ��֪����a����Ϊ����©����
(2)Ư�۹����Ũ���ᷴӦ�����Ȼ��ơ�������ˮ����ѧ����ʽΪ��Ca(ClO)2+4HCl(Ũ)�TCaCl2+2Cl2��+2H2O������Ư�ۻ�ΪKClO3�����ݵ����غ㡢ԭ���غ㣬�ɵ÷���ʽΪKClO3+6HCl(Ũ)=KCl+3Cl2��+3H2O���ɷ���ʽ��֪��ÿת��5mol���ӣ���Ӧ����3mol������������21.3gCl2�����ʵ�����0.3mol�����Է�Ӧת����0.5mol���ӣ�ת�Ƶ�����ĿΪ0.5NA��
(3)��Ӧ������Cl2�л����Ȼ����ˮ������װ��B�б���ʳ��ˮ�������dz�ȥCl2�е�HCl��װ��B������ȫƿ�����ʵ�����ʱC���Ƿ�������������������ʱB�е�����ѹǿ����ʹB��Һ����볤��©��������ƿ��Һ���½�������©���е�Һ���������γ�һ��Һ����
(4)װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ���֤�����Ƿ����Ư���ԣ�Ҫ��֤����������Ư���ԣ���ʪ�����ɫ�����У�������ˮ��Ӧ���ɴ��������Ư���ԣ�
a.IΪ�������ɫ��������ɫ������Ũ����ֻ����ˮ�֣���ͨ��ʪ�����ɫ��������ɫ������֤������Ư���ԣ���U�ι���ʢ�ŵ�Ӧ�ǹ���������a����
b.I����ʪ�����ɫ������ɫ��II�Ǹ������ˮ�Ȼ��Ʋ�������������ֻ����ˮ������III�и������ɫ��������ɫ������֤����b��ȷ��
c.IΪʪ�����ɫ������ɫ��IIΪ�������ʯ�ң���ʯ���ܹ�����ˮ������Cl2�����뵽III�������������ᷢ���κα仯��������֤��c����
�ʺ���ѡ����b��
(5)�����������廯�Ʒ�Ӧ�����嵥�ʣ�Һ���嵥�ʡ�������Cl2���ܺ͵⻯����Һ�еĵ⻯�ط�Ӧ���ɵⵥ�ʣ��ⵥ�������ڱ������Ϻ�ɫ�����ܶ�С��ˮ��������ˮ����Һ�ֲ㣻������Cl2Ҳ�ɽ�I-����ΪI2������ͨ��E����Һ��Ϊ���㣬�ϲ�(����)Ϊ�Ϻ�ɫ������˵�����������ǿ�ڵ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�