��Ŀ����
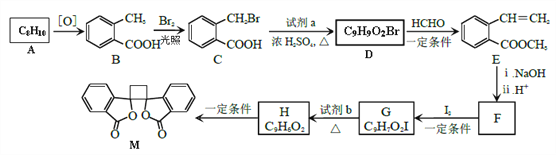
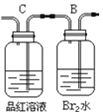
����Ŀ��ij��ѧС���������������������װ�ã���ͼ�����û������ƻ���ϩ��
��֪��![]()
�ܶȣ� | �۵�� | �е�� | �ܽ��� | |
������ | 0.096 | 25 | 161 | ������ˮ |
����ϩ | 0.081 | -103 | 83 | ������ˮ |
�Ʊ���Ʒ��
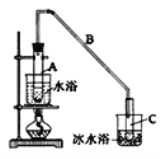
��12.5mL�����������Թ�A�У��ټ���lmLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��1��A�д�Ƭ��������______������B���˵�������е�������______��
��2���Թ�C���ڱ�ˮԡ�е�Ŀ����__________________________��
�Ʊ���Ʒ��
��3������ϩ��Ʒ�к��л������������������ʵȡ���������ʳ��ˮ�������á��ֲ㣬����ϩ��_______�㣨����������������������Һ����_________�������ţ�ϴ�ӣ�
a��KMnO4��Һ b��ϡH2SO4 c��Na2CO3��Һ
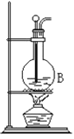
��4���ٽ�����ϩ��ͼ2װ��������ȴˮ��_______����f��g���ڽ��룬����ʱҪ������ʯ�ң�Ŀ����______________���ռ���Ʒʱ���¶�Ӧ������_____���ҡ�

��5���������ֻ���ϩ��Ʒ���Ʒ�ķ�������������_________��
a�������Ը��������Һ b���ý����� c���ⶨ�е�
��������װ���Ʊ������������ش��������⡣
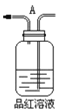
��6���÷�Һ©���ᴿ�Թ�C�е������������ڲ���ʱҪע����ϴ�Ӻ��÷�Һǰ����������������������__________�����ţ�
a�������������ڼ��ӻ���Ȧ�ϴ��ϲ�����
b�������������ڼ��ӻ���Ȧ�ϴ���
c�������ĵ�ס�����ϲ����ӵ��ú����
d����������ƽ����ʵ��̨�ϴ���
��7�����Ƶõ���Ӧ�Ӹ÷�Һ©����__________�����ţ�
a���²����� b���Ͽڵ��� c��������
��8�����ᴿ��������ʱ��ΪʲôҪʹ�ñ���̼������Һ��������NaOH��Һϴ�ӣ�
______________________________________��
���𰸡���ֹ���� ���� ��һ����ȴ����ֹ����ϩ�ӷ� �ϲ� c g ��ȥ�˲�����ˮ 83�� bc c b ��Ϊ����������Һ��ʹ������������ˮ�⣬�������ռ�
��������
������������ȥ��Ӧ���ɻ���ϩ����Ӧˮԡ���ȣ�����ϩ�뻷�����е���ϴ����������з��룬�¶ȿ����ڻ���ϩ�е㸽����
��1��A�д�Ƭ���ɶ�ף������Ƿ�ֹҺ�屬�У�����B�����������ܳ��������������ֽӴ������е�������������
��2�����������۵���������ϣ���ǰ��ֻ�����������£��Թ�C���ڱ�ˮԡ�е�Ŀ���ǽ�һ����ȴ����ֹ����ϩ�ӷ���
��3��������������ˮ������ϩ���ܶȱ�ˮС�����ϲ㣬��Һ���������ʿ�����Na2CO3��Һ��ȥ��������ػὫ����ϩ������ϡ���������������ʣ�
��4����������ԭ����ȴˮ��g�ڽ��룬����ʱҪ������ʯ�ң���ʯ����ˮ��Ӧ����ȥ�˲�����ˮ���ռ���Ʒʱ���¶�Ӧ������83�����ҡ�
��5������ϩ��Ʒ���Ʒ��������Ҫ���ڻ������Ĵ��ڣ���ͨ�����黷�������ж���Ʒ״̬��
a�������Ը��������Һ���뻷��ϩ��Ӧ�����������ߣ�a����
b���ý��������뻷������Ӧ������������b��ȷ��
c��ͨ���ⶨ�е�����ж��������ʣ�c��ȷ��
��Ϊbc��
��6���÷�Һ©���ᴿ�Թ�C�е������������ڲ���ʱҪע����ϴ�Ӻ��÷�Һǰ���������������������������ĵ�ס�����ϲ����ӵ��ú������
��7�����Ƶõ������ϲ㣬Ӧ�Ӹ÷�Һ©�����Ͽڵ�����
��8�����ᴿ��������ʱ��Ҫʹ�ñ���̼������Һ����Ϊ����������Һ��ʹ������������ˮ�⣬�������ռ���

����Ŀ�����仯������������;��������ʵ��������������ʾ��
H2S | S8 | FeS2 | SO2 | SO3 | H2SO4 | |
�۵�/�� | -85.5 | 115.2 | >600(�ֽ�) | 75.5 | 16.8 | 10.3 |
�е�/�� | -60.3 | 444.6 | -10.0 | 45.0 | 337.0 |
�ش��������⣺
(1)��̬Feԭ�Ӽ۲���ӵĵ����Ų�ͼ(�������ʽ)Ϊ______����̬Sԭ�ӵ���ռ������ܼ��ĵ���������ͼΪ______�Ρ�
(2)���ݼ۲���ӶԻ������ۣ�H2S��SO2��SO3����̬�����У�����ԭ�Ӽ۲���Ӷ�����ͬ���������ӵ���______��
(3)ͼ(a)ΪS8�Ľṹ�����۵�ͷе�Ҫ�ȶ���������۵�ͷе�ߺܶ࣬��Ҫԭ��Ϊ____��

(4)��̬���������Ե�������ʽ���ڣ�����ӵ����幹��Ϊ______�Σ����й��ۼ���������______�֣��������������д�����ͼ(b)��ʾ�����۷��ӡ��÷�����Sԭ�ӵ��ӻ��������Ϊ______��
(5)FeS2����ľ�����ͼ(c)��ʾ���þ�����FeS2����___________�������߳�Ϊanm��FeS2���ʽ��ΪM�������ӵ�������ֵΪNA���侧���ܶȵļ������ʽΪ_____g/cm3.(д������ʽ) ��������Fe2+λ��S22-���γɵİ���������ģ�����������ı߳�Ϊ______nm��
����Ŀ��ijС����Fe2+����ʵ���й۲쵽�쳣����Ϊ̽������ɫ��ȥ����ԭ��������ʵ�飺
��� | ʵ��I | ʵ��II | ʵ��III |
ʵ�鲽�� |
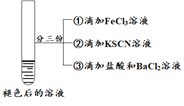
| ��ʵ��I��ɫ�����Һ�����ݷֱ����ʵ��
| Ϊ��һ��̽������ɫ��ȥ����ԭ���ֽ�������ʵ�� �� ��ȡ��Ӧ�����Һ���μ������BaCl2��Һ |
���� | ��Һ�ȱ�죬Ƭ�̺��ɫ��ȥ�����������ɣ�������ΪO2�� | ������������ ����Һ��� �۲�����ɫ���� | ����Һ��죬һ��ʱ�����ɫ�� ���ް�ɫ�������� |
������������ʵ�飬������������ȷ����
A.�ڴ�ʵ��������H2O2����Fe2�������ʱ�����SCN�������ʿ�
B.ͨ��ʵ����Ƴ�ʵ����к�ɫ��ȥ��ԭ��������SCN��������
C.ͨ��ʵ����ʵ���Ա��Ƴ���ɫ��ȥֻ��H2O2���������й�
D.����������ʵ����к�ɫ��ȥ��ԭ���뻯ѧƽ���ƶ�ԭ����