��Ŀ����
����Ŀ��ij̼������Ʒ�п��ܺ����������ơ�̼��ơ���ʯ�ҡ��Ȼ��ơ�����ͭ���������е����֡��ֽ�������ʵ�飺
�ٳ�ȡ4.7g ��Ʒ����������ˮ����Ʒ�����ܽ⡣
���������������Һ�м���100mL1 mol/LHCl�����յõ���ɫ������Һ�� �˹����й�����0.04 mol���塣
������з�Ӧ����Һ�м�����������������ϡ���ᣬ�õ�15.8g��ɫ������
�ɴ˿�֪�����У� ��
A.һ����NaCl��һ������CuSO4B.���ܺ�CaO��NaOH
C.һ����CaCO3�����ܺ�NaOHD.���ܺ�CaO������CaCO3
���𰸡�AC
��������
�������������Һ�м���100mL1 mol/LHCl�����յõ���ɫ������Һ����֪��Ʒ�в���CuSO4���˹����й�����0.04 mol���壬��CO2����CO32-+2H+=CO2+H2O��֪��Ӧ������n(HCl)=2n(CO2)=0.08mol����HCl��ʣ�࣬CO32-��ȫ��Ӧ����ȡ4.7g ��Ʒ����������ˮ����Ʒ�����ܽ⣬��֪��Ʒ�к���CaCO3��CaCO3��CaO��CaO�����������һ�֣�����з�Ӧ����Һ�м�����������������ϡ���ᣬ�õ�15.8g��ɫ��������AgCl��n(AgCl)=![]() >0.1mol����ԭ��Ʒ��һ����NaCl������Ϊ(
>0.1mol����ԭ��Ʒ��һ����NaCl������Ϊ(![]() -0.1mol)��58.5g/mol
-0.1mol)��58.5g/mol![]() 0.59g����ԭ��Ʒ�������ɷ�������Ϊ4.7g-0.59g=4.11g��������CaCO3����̼Ԫ�����ʵ���<
0.59g����ԭ��Ʒ�������ɷ�������Ϊ4.7g-0.59g=4.11g��������CaCO3����̼Ԫ�����ʵ���<![]() �����������⣬��ԭ��Ʒ��һ������CaCO3������ԭ��Ʒֻ���������ʣ���CaO��NaOHֻ������һ�֡�
�����������⣬��ԭ��Ʒ��һ������CaCO3������ԭ��Ʒֻ���������ʣ���CaO��NaOHֻ������һ�֡�
�������������Һ�м���100mL1 mol/LHCl�����յõ���ɫ������Һ����֪��Ʒ�в���CuSO4���˹����й�����0.04 mol���壬��CO2����CO32-+2H+=CO2+H2O��֪��Ӧ������n(HCl)=2n(CO2)=0.08mol����HCl��ʣ�࣬CO32-��ȫ��Ӧ����ȡ4.7g ��Ʒ����������ˮ����Ʒ�����ܽ⣬��֪��Ʒ�к���CaCO3��CaCO3��CaO��CaO�����������һ�֣�����з�Ӧ����Һ�м�����������������ϡ���ᣬ�õ�15.8g��ɫ��������AgCl��n(AgCl)=![]() >0.1mol����ԭ��Ʒ��һ����NaCl������Ϊ(
>0.1mol����ԭ��Ʒ��һ����NaCl������Ϊ(![]() -0.1mol)��58.5g/mol
-0.1mol)��58.5g/mol![]() 0.59g����ԭ��Ʒ�������ɷ�������Ϊ4.7g-0.59g=4.11g��������CaCO3��̼Ԫ�����ʵ���<
0.59g����ԭ��Ʒ�������ɷ�������Ϊ4.7g-0.59g=4.11g��������CaCO3��̼Ԫ�����ʵ���<![]() �����������⣬��ԭ��Ʒ��һ������CaCO3������ԭ��Ʒֻ���������ʣ���CaO��NaOHֻ������һ�֣�
�����������⣬��ԭ��Ʒ��һ������CaCO3������ԭ��Ʒֻ���������ʣ���CaO��NaOHֻ������һ�֣�
A���ɷ�����֪��ԭ��Ʒ��һ����NaCl��һ������CuSO4����A��ȷ��
B���ɷ�����֪��ԭ��Ʒ�в�����ͬʱ��CaO��NaOH����B����
C���ɷ�����֪��ԭ��Ʒ��һ����CaCO3�����ܺ�NaOH����C��ȷ��
D���ɷ�����֪��ԭ��Ʒ�п��ܺ�CaO��һ������CaCO3����D����
�ʴ�ѡAC��

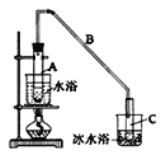
����Ŀ��ij��ѧС���������������������װ�ã���ͼ�����û������ƻ���ϩ��
��֪��![]()
�ܶȣ� | �۵�� | �е�� | �ܽ��� | |
������ | 0.096 | 25 | 161 | ������ˮ |
����ϩ | 0.081 | -103 | 83 | ������ˮ |
�Ʊ���Ʒ��
��12.5mL�����������Թ�A�У��ټ���lmLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��1��A�д�Ƭ��������______������B���˵�������е�������______��
��2���Թ�C���ڱ�ˮԡ�е�Ŀ����__________________________��
�Ʊ���Ʒ��
��3������ϩ��Ʒ�к��л������������������ʵȡ���������ʳ��ˮ�������á��ֲ㣬����ϩ��_______�㣨����������������������Һ����_________�������ţ�ϴ�ӣ�
a��KMnO4��Һ b��ϡH2SO4 c��Na2CO3��Һ
��4���ٽ�����ϩ��ͼ2װ��������ȴˮ��_______����f��g���ڽ��룬����ʱҪ������ʯ�ң�Ŀ����______________���ռ���Ʒʱ���¶�Ӧ������_____���ҡ�
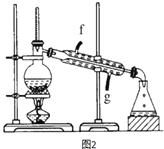
��5���������ֻ���ϩ��Ʒ���Ʒ�ķ�������������_________��
a�������Ը��������Һ b���ý����� c���ⶨ�е�
��������װ���Ʊ������������ش��������⡣
��6���÷�Һ©���ᴿ�Թ�C�е������������ڲ���ʱҪע����ϴ�Ӻ��÷�Һǰ����������������������__________�����ţ�
a�������������ڼ��ӻ���Ȧ�ϴ��ϲ�����
b�������������ڼ��ӻ���Ȧ�ϴ���
c�������ĵ�ס�����ϲ����ӵ��ú����
d����������ƽ����ʵ��̨�ϴ���
��7�����Ƶõ���Ӧ�Ӹ÷�Һ©����__________�����ţ�
a���²����� b���Ͽڵ��� c��������
��8�����ᴿ��������ʱ��ΪʲôҪʹ�ñ���̼������Һ��������NaOH��Һϴ�ӣ�
______________________________________��