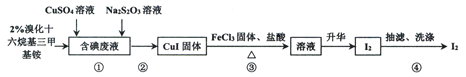
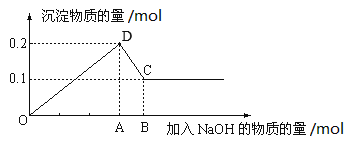
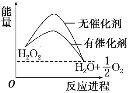
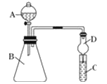
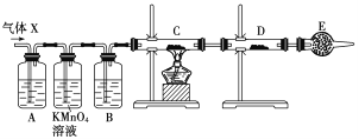
��Ŀ����
����Ŀ������ɫ����X�����ֳ�����Ԫ����ɣ�ʽ��Ϊ412����������ˮ�����ֽ⣬���±�ը��
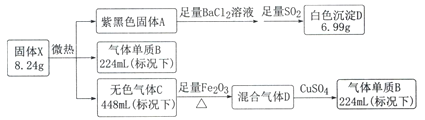
��֪������B�ڱ�״���µ��ܶ�Ϊ1.25g.L-1���������ͨ��CuSO4��CuSO4�����Ϊ��ɫ��
��ش��������⣺
��1��д��A�ĵ���ʽ____________��
��2��д�����ɰ�ɫ����D�Ļ�ѧ����ʽ________________________��
��3������X����A���������C��Ũ��Һ��Ӧ���ɣ������ӷ���ʽΪ_________________
��4�������������C�ڼ�����������Fe2O3��Ӧ�����о���������в���+3�۵���Ԫ�أ������ʵ�鷽�������������п��ܵijɷ֣����û�ѧ������________________________
���𰸡�![]() SO2+I2+BaCl2+2H2O=BaSO4��+2HI+2HCl 3I2+5NH3��H2O=NI3��NH3+3NH4++3I-+5H2O ȡ���������������������������ͭ��Һ����ַ�Ӧ�����к�ɫ������֣�֤���������������õ���������ϡ���ᣬ�μ����軯����Һ�������ٵμ���ˮ������Һ�ʺ�ɫ����֤����������������
SO2+I2+BaCl2+2H2O=BaSO4��+2HI+2HCl 3I2+5NH3��H2O=NI3��NH3+3NH4++3I-+5H2O ȡ���������������������������ͭ��Һ����ַ�Ӧ�����к�ɫ������֣�֤���������������õ���������ϡ���ᣬ�μ����軯����Һ�������ٵμ���ˮ������Һ�ʺ�ɫ����֤����������������
��������
����B�ڱ�״���µ��ܶ�Ϊ1.25g.L-1������Ħ������Ϊ22.4L/mol��1.25 g.L-1=28 g/mol��Ϊ�������������ͨ��CuSO4��CuSO4�����Ϊ��ɫ��˵����������к���ˮ�����͵���������ǰ�����������仯��������ɫ����CΪ�������Ϻ�ɫ����AӦΪ�ⵥ�ʣ��������������Ȼ�����Ӧ�������ᱵ����������ɫ����6.99��Ϊ���ᱵ��������0.03mol��ͨ�����Ӽ���ⵥ�ʵ����ʵ���Ϊ0.03mol�����������ʵ���Ϊ0.01mol�����������ʵ���Ϊ0.02mol�������������ʵ�������Ϊ8.24�ˣ������ǹ���X������������X�Ļ�ѧʽΪNI3��NH3��
��1��AΪ�ⵥ�ʣ�����ʽΪ��![]() ��
��
��2���ⵥ�ʺͶ���������Ȼ�����ˮ��Ӧ�������ᱵ�����͵⻯������ᣬ����ʽΪ��SO2+I2+BaCl2+2H2O=BaSO4��+2HI+2HCl��
��3������X���ɵ���������就����Ũ��Һ��Ӧ���ɣ����ӷ���ʽΪ��3I2+5NH3��H2O=NI3��NH3+3NH4++3I-+5H2O ��
��4����������в���+3�۵���Ԫ�أ����Է�Ӧ����ܲ�����������������������������ͭ��Ӧ�û�����ɫ����ͭ�����Ƿ����������������ļ�����������������������軯���Ժ�ɫ�����ʽ��У���ʵ�����Ϊ��ȡ���������������������������ͭ��Һ����ַ�Ӧ�����к�ɫ������֣�֤���������������õ���������ϡ���ᣬ�μ����軯����Һ�������ٵμ���ˮ������Һ�ʺ�ɫ����֤����������������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�