ЬтФПФкШн
ЁОЬтФПЁПвРОнЬтвтЃЌЭъГЩЯТСаИїЬтЃК
(1)1.0 gввДМЭъШЋШМЩеЩњГЩвКЬЌЫЎЗХГі1.37 kJШШСПЃЌБэЪОввДМЕФШМЩеШШЕФШШЛЏбЇЗНГЬЪНЮЊ__________________________________________________;
(2) ЁАГЄеїЁБ2КХЛ№М§ЕФЗЂЖЏЛњжагУЁАЦЋЖўМзыТЁБЃЈЗжзгЪНЮЊC2H8N2ЃЉКЭЫФбѕЛЏЖўЕЊзїЮЊвКЬЌШМСЯЁЃвбжЊa gЦЋЖўМзыТгыb gЫФбѕЛЏЖўЕЊдкЗЂЖЏЛњФкШМЩеЩњГЩЮШЖЈЕФЁЂЖдЛЗОГгбКУЕФЮяжЪЁЃШєЩњГЩ1 mol N2(g) ЕФЗДгІШШЮЊc kJЃЌаДГіШШЛЏбЇЗНГЬЪН______________________________________________;
(3) T ЁцЪБЃЌШчЭМЫљЪОЃЌ ЖдгІЕФЛЏбЇЗНГЬЪНЮЊ______________________;
(4) ЯђзуСПЕФH2SO4ШмвКжаМгШы100 mL 0.4 molЁЄLЃ1ЕФBa(OH)2ШмвКЃЌЗХГіЕФШШСПЪЧ5.12 kJЁЃЯђзуСПЕФBa(OH)2ШмвКжаМгШы100 mL 0.4 molЁЄLЃ1ЕФHClШмвКЃЌЗХГіЕФШШСПЮЊ2.2 kJЁЃдђNa2SO4ШмвКгыBaCl2ШмвКЗДгІЕФШШЛЏбЇЗНГЬЪНЮЊBa2ЃЋ(aq)ЃЋSO42-(aq)===BaSO4(s) ІЄHЃН______________;
(5) баОПБэУїЃЌЛЏбЇЗДгІЕФФмСПБфЛЏ(ІЄH)гыЗДгІЮяКЭЩњГЩЮяЕФМќФмгаЙиЁЃМќФмПЩвдМђЕЅЕиРэНтЮЊЖЯПЊ1 molЛЏбЇМќЪБЫљашЮќЪеЕФФмСПЁЃЯТБэЪЧВПЗжЛЏбЇМќЕФМќФмЪ§ОнЃК
ЛЏбЇМќ | PЁЊP | PЁЊO | O==O | P==O |
МќФмkJ/mol | 197 | 360 | 499 | x |
вбжЊАзСз(P4)ЕФШМЩеШШЮЊ2378.0 kJ/molЃЌАзСзЭъШЋШМЩеЕФВњЮя(P4O10)ЕФНсЙЙШчЭМЫљЪОЃЌдђЩЯБэжаxЃН_________________ЁЃ


ЁОД№АИЁПC2H5OH(l)ЃЋ3O2(g)===2CO2(g)ЃЋ3H2O(l)ІЄHЃНЃ63.02 kJЁЄmolЃ1C2H8N2(l)ЃЋ2N2O4(l)===2CO2(g)ЃЋ3N2(g)ЃЋ4H2O(l) ІЄHЃН-3c kJЁЄmolЃ1A(g)ЃЋ3B(g) ![]() 2C(g)Ѓ18 kJЁЄmolЃ1433.75 kJЁЄmolЃ1
2C(g)Ѓ18 kJЁЄmolЃ1433.75 kJЁЄmolЃ1
ЁОНтЮіЁП
(1)1.0 gввДМЭъШЋШМЩеЩњГЩвКЬЌЫЎЗХГі1.37 kJШШСПЃЌ46.0 gввДМЭъШЋШМЩеЩњГЩвКЬЌЫЎЗХГі 63.02kJШШСП, БэЪОввДМЕФШМЩеШШЕФШШЛЏбЇЗНГЬЪНЮЊ: C2H5OH(l)ЃЋ3O2(g)===2CO2(g)ЃЋ3H2O(l)ІЄHЃНЃ63.02 kJЁЄmolЃ1ЃЛзлЩЯЫљЪіЃЌБОЬтД№АИЪЧЃКC2H5OH(l)ЃЋ3O2(g)===2CO2(g)ЃЋ3H2O(l)ІЄHЃНЃ63.02 kJЁЄmolЃ1ЁЃ
(2)гЩЬтвтПЩжЊ C2H8N2гыN2O4ПЩвдЗЂЩњбѕЛЏЛЙдЗДгІЃЌЗДгІЗНГЬЪНC2H8N2ЃЋ2N2O4=2CO2ЃЋ4H2OЃЋ3N2ЃЛОнЗДгІПЩжЊЩњГЩ3molN2ЪБЗХГіШШСПЮЊ3ckJЃЌвђДЫШШЛЏбЇЗДгІЗНГЬЪНЮЊC2H8N2(l)ЃЋ2N2O4(l)ЃН2CO2(g)ЃЋ3N2(g)ЃЋ4H2O(l)ЁЁІЄHЃНЃ3c kJЁЄmolЃ1ЃЛзлЩЯЫљЪіЃЌБОЬтД№АИЪЧЃКC2H8N2(l)ЃЋ2N2O4(l)===2CO2(g)ЃЋ3N2(g)ЃЋ4H2O(l) ІЄHЃН-3c kJЁЄmolЃ1ЁЃ
(3) T ЁцЪБЃЌЭЈЙ§ЭМЪОПЩжЊЃЌA ЁЂBЕФХЈЖШОљМѕаЁЃЌCЕФХЈЖШдіДѓЃЌA ЁЂBЮЊЗДгІЮяЃЌCЮЊЩњГЩЮяЃЛA МѕаЁ0.2mol/LЃЌBМѕаЁ0.6mol/LЃЌCдіМг0.4 mol/LЃЌЯЕЪ§БШКЭХЈЖШЕФБфЛЏСПГЩе§БШЃЌвђДЫЖдгІЕФЛЏбЇЗНГЬЪНЮЊЃКA(g)ЃЋ3B(g) ![]() 2C(g)ЃЛзлЩЯЫљЪіЃЌБОЬтД№АИЪЧЃКA(g)ЃЋ3B(g)
2C(g)ЃЛзлЩЯЫљЪіЃЌБОЬтД№АИЪЧЃКA(g)ЃЋ3B(g) ![]() 2C(g)ЁЃ
2C(g)ЁЃ
ЃЈ4ЃЉ100 mL 0.4 molЁЄLЃ1ЕФBa(OH)2ШмвКжаBa(OH)2ЕФЮяжЪЕФСПЮЊ0.04molЃЌзуСПH2SO4ШмвКжаМгШы100 mL 0.4 molЁЄLЃ1ЕФBa(OH)2ШмвКЃЌЗХГіЕФШШСПЪЧ5.12 kJ ЃЌдђ1 mol Ba(OH)2 ВЮгыЗДгІЪБЃЌЗХГіЕФШШСПЮЊ5.12/0.04=128 kJ ЃЌдђгаЂй2H+(aq)+SO42-(aq)+Ba2+(aq)+2OH-(aq)=BaSO4(s)+2H2O(l)ЃЌІЄH1 =-128 kJЁЄmolЃ1ЃЛ100 mL 0.4 molЁЄLЃ1бЮЫсжаHClЕФЮяжЪЕФСПЮЊ0.04molЃЌзуСПBa(OH)2ШмвКжаМгШы100 mL 0.4 molЁЄLЃ1бЮЫсЪБЃЌЗХГіЕФШШСПЮЊ2.2 kJ ЃЌдђ2molHCl ВЮгыЗДгІЪБЗХГіЕФШШСПЮЊ2ЁС2.2/0.04=110 kJЁЄmolЃ1ЃЌдђгаЂк2H+(aq)+ 2OH-(aq)=2H2O(l)ЃЌІЄH2 =-110 kJЁЄmolЃ1ЃЌИљОнИЧЫЙЖЈТЩЂй-ЂкПЩЕУЃЌSO42-(aq)+Ba2+(aq)= BaSO4(s) ІЄH=ІЄH1-ІЄH2=-18 kJЁЄmolЃ1ЃЛзлЩЯЫљЪіЃЌБОЬтД№АИЪЧЃКЃ18 kJЁЄmolЃ1 ЁЃ
(5) АзСзШМЩеЕФЗНГЬЪНЮЊP4+5O2===P4O10 ІЄH =-2378 kJЁЄmolЃ1ЃЌ1molАзСзЭъШЋШМЩеашВ№ПЊ6molP-PЁЂ5molO=OЃЌаЮГЩ12molP-OЁЂ4molP=O,Ыљвд12molЁС360kJ/mol+4molЁСxkJ/mol-ЃЈ6molЁС197 kJ/mol+5 molЁС499 kJ/molЃЉ=2378.0kJЃЌx=433.75ЃЛзлЩЯЫљЪіЃЌБОЬтД№АИЪЧЃК433.75 kJЁЄmolЃ1ЁЃ

ЁОЬтФПЁПФГЛЏбЇаЫШЄаЁзщНјаавдЯТЪЕбщЬНОПЃК


Ђё. ЩшМЦЪЕбщЬНОПЗДгІЫйТЪЕФВтЖЈКЭБШНЯ
ЪЕбщВНжшЃК
(1)ШЁвЛЬззАжУЃЈзАжУШчЭМЫљЪОЃЉЃЌМгШы40 mL 1 molЁЄLЃ1ЕФСђЫсЃЌВтСПЪеМЏ10 mL H2ЫљашЕФЪБМфЁЃ
(2)ШЁСэвЛЬззАжУЃЌМгШы40 mL 4 molЁЄLЃ1ЕФСђЫсЃЌВтСПЪеМЏ10 mL H2ЫљашЕФЪБМфЁЃ
ЪЕбщЯжЯѓЃК аПИњСђЫсЗДгІВњЩњЦјХнЃЌЪеМЏ10 mLЦјЬхЃЌЃЈ2ЃЉЫљгУЪБМфБШЃЈ1ЃЉЫљгУЪБМф________ЃЈЬюЁАГЄЁБЛђЁАЖЬЁБЃЉЃЛ
ЪЕбщНсТлЃК 4 molЁЄLЃ1СђЫсгыаПЗДгІБШ1 molЁЄLЃ1СђЫсгыаПЗДгІЫйТЪ______ЃЈЬюЁАДѓЁБЛђЁАаЁЁБЃЉЁЃ
зЂвтЪТЯюЃКЂй аПСЃЕФПХСЃЃЈМДБэУцЛ§ЃЉДѓаЁ________________ЃЛ
Ђк 40 mLЕФСђЫсвЊбИЫйМгШыЃЛ
Ђл зАжУ____________________ЃЌЧвМЦЪБвЊбИЫйзМШЗЃЛ
Ђм ЦјЬхЪеМЏПЩвдгУХХЫЎСПЦјзАжУДњЬцЁЃ
ЪЕбщЬжТлЃКГ§БОЪЕбщВтЖЈЗДгІЫйТЪЕФЗНЗЈЭтЃЌПЩааЕФЗНАИЛЙгаЃЈШЮаДвЛжжЃЉ
________________________________________________________________ЁЃ
Ђђ. ЬНОПгУHNO3гыДѓРэЪЏЗДгІЙ§ГЬжажЪСПМѕаЁЕФЗНЗЈЃЌбаОПгАЯьЗДгІЫйТЪЕФвђЫиЃЌЫљгУHNO3ХЈЖШЮЊ1.00 molЁЄLЃ1ЁЂ2.00 molЁЄLЃ1ЃЌДѓРэЪЏгаЯИПХСЃгыДжПХСЃСНжжЙцИёЃЌЪЕбщЮТЖШЮЊ298 KЁЂ308 KЁЃ
ЧыЭъГЩвдЯТЪЕбщЩшМЦБэЃЌВЂдкЪЕбщФПЕФвЛРИжаЬюГіЖдгІЕФЪЕбщБрКХЃК
ЪЕбщБрКХ | T(K) | ДѓРэЪЏЙцИё | HNO3ХЈЖШ (molЁЄLЃ1) | ЪЕбщФПЕФ |
Ђй | 298 | ДжПХСЃ | 2.00 | ЃЈ1ЃЉЪЕбщЂйКЭЂкЬНОПHNO3ХЈЖШЖдИУЗДгІЫйТЪЕФгАЯь ЃЈ2ЃЉЪЕбщЂйКЭ_____ЬНОПЮТЖШЖдИУЗДгІЫйТЪЕФгАЯь ЃЈ3ЃЉЪЕбщЂйКЭ_____ЬНОПДѓРэЪЏЙцИё(ДжЁЂЯИ)ЖдИУЗДгІЫйТЪЕФгАЯь |
Ђк | _____ | ________ | _________ | |
Ђл | _____ | ДжПХСЃ | _________ | |
Ђм | ______ | ______ | ________ |
ЁОЬтФПЁПКЃбѓзЪдДЕФРћгУОпгаЙуРЋЧАОАЁЃ
ЃЈ1ЃЉЯТЭМЪЧДгКЃЫЎжаЬсШЁУОЕФМђЕЅСїГЬЁЃ
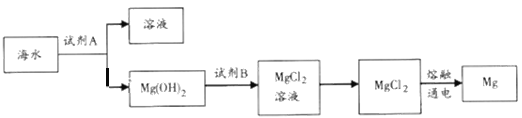
ЂйЙЄвЕЩЯГЃгУгкГСЕэMg2+ЕФЪдМСAЪЧ__________ЃЈЬюЛЏбЇЪНЃЉЃЌMg(OH)2зЊЛЏЮЊMgCl2ЕФРызгЗНГЬЪНЮЊ________________________ЁЃ
ЂкгЩЮоЫЎMgCl2жЦШЁMgЕФЛЏбЇЗНГЬЪНЪЧ______________________ЁЃ
ЃЈ2ЃЉКЃДјЛвжаИЛКЌвдIЃаЮЪНДцдкЕФЕтдЊЫиЃЌЪЕбщЪвЬсШЁI2ЕФЭООЖШчЯТЫљЪОЃК
![]()
ЂйзЦЩеКЃДјжСЛвН§ЪБЫљгУЕФжївЊвЧЦїЪЧ__________ЃЈЬюУћГЦЃЉЃЛ
ЂкЯђЫсЛЏЕФТЫвКжаМгЙ§бѕЛЏЧтШмвКЃЌИУЗДгІЕФРызгЗНГЬЪНЮЊ__________ЃЛ
ЂлЗДгІНсЪјКѓЃЌМгШыCCl4зїнЭШЁМС,ВЩгУнЭШЁЃЗжвКЕФЗНЗЈДгЕтЫЎжаЬсШЁЕтЃЌжївЊВйзїВНжшШчЯТЭМЃК
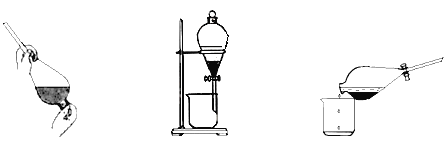
Мз.МгШынЭШЁМСКѓеёЕД вв.ОВжУЗжВу Бћ.ЗжРы
МзЁЂввЁЂБћ3ВНЪЕбщВйзїжаЃЌДэЮѓЕФЪЧ__________ЃЈЬюЁАМзЁБЁЂЁАввЁБЛђЁАБћЁБЃЉЁЃ
ЃЈ3ЃЉКЃЫЎжаВПЗжРызгЕФКЌСПШчЯТ:
ГЩЗж | КЌСПЃЈmg/LЃЉ | ГЩЗж | КЌСПЃЈmg/LЃЉ |
Na+ | 10560 | ClЃ | 18980 |
Mg2+ | 1272 | BrЃ | 64 |
Ca2+ | 400 | SO42Ѓ | 2560 |
ШєДг100LИУКЃЫЎжаЬсШЁУОЃЌРэТлЩЯашМгШыЪдМСA__________gЁЃ
ЁОЬтФПЁПToCЯТЃЌЯђЬхЛ§ЮЊ2 LЕФКуШнУмБеШнЦїжаЭЈШыNO2КЭO2ЃЌЗЂЩњЗДгІЃК4NO2(g)+O2(g)![]() 2N2O5(g)ІЄHЃМ0ЃЌВПЗжЪЕбщЪ§ОнШчЯТБэЁЃЯТСаЫЕЗЈВЛе§ШЗЕФЪЧ
2N2O5(g)ІЄHЃМ0ЃЌВПЗжЪЕбщЪ§ОнШчЯТБэЁЃЯТСаЫЕЗЈВЛе§ШЗЕФЪЧ
ЪБМф/s | 0 | 5 | 10 | 15 |
n(NO2)/mol | 8.00 | n1 | n2 | 4.00 |
n(O2)/mol | 2.00 | 1.25 | 1.00 | n3 |
A. 5sФкNO2ЕФЦНОљЗДгІЫйТЪЮЊ0.3 mol/(LsЃЉ
B. Шє10 sЪБЃЌдйЯђШнЦїжаГфШы2 mol N2O5(g)ЃЌдђаТЦНКтЯТЃЌNO2ЕФЬхЛ§ЗжЪ§НЋдіДѓ
C. Шє5 s ЪБЃЌИФдкОјШШКуШнЯТДяЦНКтЃЌаТЦНКтЯТЕФЦНКтГЃЪ§БШдЦНКтЕФаЁ
D. ToCЃЌИУЗДгІЕФЦНКтГЃЪ§ЮЊ0.125ЃЌЗДгІЮяЕФЦНКтзЊЛЏТЪОљЮЊ50%