��Ŀ����
����Ŀ����֪����Fe+H2SO4(ϡ)=FeSO4+H2������Cu+2H2SO4(Ũ)![]() CuSO4+2H2O+SO2������KClO3+6HCl(Ũ)=KCl+3Cl2��+3H2O���Իش��������⣺
CuSO4+2H2O+SO2������KClO3+6HCl(Ũ)=KCl+3Cl2��+3H2O���Իش��������⣺
(1)����Ӧ��ת��3mol����ʱ���������������(��״��)__L��
(2)��Ӧ����__����������__���������
(3)����Ӧ��������11.2LSO2����(��״����)ʱ������ԭ��H2SO4�����ʵ�����__��
(4)�õ����ŷ���ʾ��Ӧ������ת�Ƶķ������Ŀ__(�ڻ�ѧ����ʽ�ϱ��)��Cu+2H2SO4(Ũ)![]() CuSO4+2H2O+SO2����
CuSO4+2H2O+SO2����
(5)��Ӧ�������������뻹ԭ��������ʵ���֮��Ϊ__��
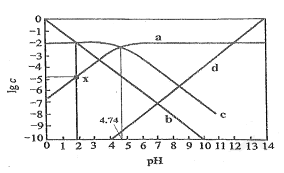
���𰸡�33.6 H2SO4(Ũ) CuSO4 0.5mol 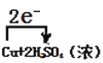
![]() CuSO4+2H2O+SO2�� 5��1
CuSO4+2H2O+SO2�� 5��1
��������
(1)Fe+H2SO4(ϡ)=FeSO4+H2��������Ӧ��ת��3Ħ������ʱ���������������(��״��)3/2mol��22.4L/mol=33.6L��
�ʴ�Ϊ��33.6��
(2)Cu+2H2SO4(Ũ)![]() CuSO4+2H2O+SO2�������������ϼ۽��ͣ�����Ũ����Ϊ����������ԭ����ʧ������Ӧ�����������ͭΪ�������
CuSO4+2H2O+SO2�������������ϼ۽��ͣ�����Ũ����Ϊ����������ԭ����ʧ������Ӧ�����������ͭΪ�������
�ʴ�Ϊ��H2SO4(Ũ)��CuSO4��
(3)1molSO2���ɣ�����1mol���ᱻ��ԭ������Ӧ��������11.2LSO2����(��״����)ʱ������ԭ��H2SO4�����ʵ�����![]() =0.5mol��
=0.5mol��
�ʴ�Ϊ��0.5mol��
(4)��ͷ��ԭ��ָ����������ע��ת�Ƶ�������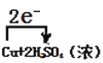
![]() CuSO4+2H2O+SO2����
CuSO4+2H2O+SO2����
�ʴ�Ϊ��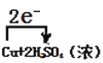
![]() CuSO4+2H2O+SO2����
CuSO4+2H2O+SO2����
(5)��Ӧ��KClO3+6HCl(Ũ)=KCl+3Cl2��+3H2O�����������뻹ԭ�����Ϊ���������ݻ�ԭ����ʧ������֪��5molCl-���������������������Ϊ5/2mol�����������û���1mol+5����������������˻�ԭ����Ϊ1/2mol�����������뻹ԭ��������ʵ���֮��Ϊ5:1��
�ʴ�Ϊ��5:1��

 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�����Ŀ����������(SOCl2)��һ����ɫ�ӷ�Һ�壬����ˮ�������������壬��������ˮ�������۵�-105�棬�е�79�棬140������ʱ�ֽ⡣
��1������ƣ�S����Һ�Ⱥ���������Ϊԭ����һ�������ϳɶ�������,ԭ�������ʴ�100%,�����ߵ����ʵ�����Ϊ______________.
��2����ͬѧ�����ͼװ����ZnCl2 xH2O������ȡ��ˮZnCl2������ʣ���SOCl2������װ��F��֤�������е�ij�����壨�гּ�����װ���ԣ���

����ԭ������SOCl2�ڸ�ʵ���е�����______________________________________�����������£�Aװ�����ܵĻ�ѧ����ʽΪ____________________.
��װ�õ�����˳��ΪA��B��_____________________��
��ʵ�������,Ϊ���ZnCl2 xH2O�����Ƿ���ȫ��ˮ����ͬѧ���ʵ�鷽�����£���ȷ��ʵ��˳��Ϊ_____________������ţ�
a.�������������ữ����������Һ����ַ�Ӧ�� b.�Ƶù���Ϊn�ˣ� c.���d.��ȡ���ɺ�Ĺ���m������ˮ��e.���ˣ�f.ϴ��
��m/n��______________������С�����һλ��������֤����������ȫ��ˮ.
��3����ͬѧ��ΪSOCl2����������FeCl3 6H2O��ȡ��ˮFeCl3����ˮ��������ͬѧ��Ϊ��ʵ����ܷ�������Ӧʹ���IJ�Ʒ������
�ٿ��ܷ����ĸ���Ӧ�����ӷ���ʽ______________________.
�ڱ�ͬѧ���������ʵ�鷽���жϸ���Ӧ�Ŀ����ԣ�
i.ȡ����FeCl3 6H2O���Թ��У���������SOCl2����ʹ�������ʳ�ַ�Ӧ��
ii.�������Թ��м�ˮ�ܽ⣬ȡ�ܽ�����Һ��������֧�Թܣ�����ʵ����֤����ɱ������ݡ�
����ѡ�Լ���AgNO3��Һ��ϡ���ᡢϡHNO3������KMnO4��Һ��BaCl2��Һ��K3[Fe(CN)6]��Һ����ˮ��
���� | ���� | ���� | ���� |
����һ | ��һ֧�Թ��еμ�_____________ | ���а�ɫ�������� | ��������������Ӧ |
������ | ����һ֧�Թ��еμ� _____________ | __________________ | ��û�з�����������Ӧ |