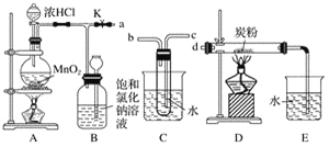
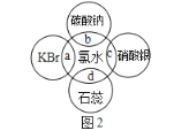
��Ŀ����
����Ŀ������˵������ȷ���ǣ� ��
A.��0.1molL-1�İ�ˮ�м�����������粒��壬��Һ��![]() ����
����
B.����Сʵ�飺�������ǽ�����ʳ���У������ݲ�����˵��CH3COOH���������
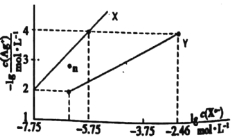
C.�мס������ִ�����Һ����ü�pH=a���ҵ�pH=a+1���������к͵����ʵ���Ũ�ȵ������NaOH��Һ�������ļס�����������V(��)>10V(��)
D.�����ͬ��Ũ�Ⱦ�Ϊ0.1molL-1��NaOH��Һ����ˮ���ֱ�ϡ��m��n����ʹ��Һ��pH����Ϊ9����m<n
���𰸡�C
��������
A.��Ϊ笠����Ӽ����һˮ�ϰ��ĵ���ƽ���ܵ����ƣ���![]() ��С����A����
������A����
B.��������̼��Ʒ�Ӧ�ų�������̼���壬˵����������Ա�̼�������ǿ��������˵�����������ᣬҲ����˵��������������ʣ���B����
C.����ԽϡԽ�����֪��c���ף�>10c���ң������������к͵����ʵ���Ũ�ȵ������NaOH��Һ�������ļס�����������V(��)>10V(��)����C��ȷ��
D.�����ͬ��Ũ�Ⱦ�Ϊ0.1molL-1��NaOH��Һ����ˮ��pH��NaOH��> pH����ˮ������ʹ��Һ��pH����Ϊ9����Ӧ������������Һ�м�������ˮ����m>n����D����
������������ΪC��

 Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�����Ŀ���±���Ԫ�����ڱ���һ���֣���Ա��еĢ١�����Ԫ�أ����û�ѧ����ش��������⣺
�� ���� | IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | �� | |||
4 | �� | �� |
��1�� �ڢۡ���Ԫ���У�ԭ�Ӱ뾶������__________����Ԫ�ط��ţ���
��2�� ��Ԫ�ص�����������Ӧ��ˮ���������⻯����������M��M�к��еĻ�ѧ��������________________________��
��3��д��Ԫ�آٺ͢�ĵ����ڼ��������·�Ӧ���ɵĻ�����ĵ���ʽ��_____________��
��4�� �ۡ��ݡ��ߡ����γɵ����ӣ���뾶��С�����˳����________�������ӷ��ţ�
��5�� �١�����Ԫ������������Ӧ��ˮ������������ǿ����_____________�������ʻ�ѧʽ���������Ե�����������_________�������ʻ�ѧʽ�����û�������NaOH��Һ��Ӧ�����ӷ���ʽΪ___________��
��6�� �õ���ʽ��ʾԪ�آ�����γɻ�����Ĺ���_____________________________��
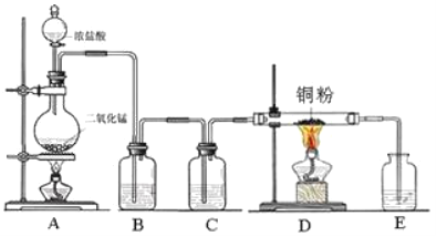
��7��д����ҵұ���ݵĻ�ѧ����ʽ��_______________________________________��