��Ŀ����
����Ŀ��NH4Al(SO4)2��NH4HSO4��;�㷺����ش��������⣺
��1������ʱ��0.1 mol��L-1 NH4Al(SO4)2��Һ��pH=3������Һ��c(NH4��)+c(NH3��H2O)_______c (Al3+) + c��Al(OH)3�ݣ����������������=������2c(SO42��)- c(NH4��)-3c(Al3+)=________mol��L-1������ֵ����
��2��80��ʱ��0.1 mol��L-1 NH4Al(SO4)2��Һ��pHС��3����������pH���¶ȱ仯��ԭ����________________________ �������ӷ���ʽ��������������ش𣩡�
��3������ʱ����100 mL 0.1 mol��L-1 NH4HSO4��Һ�еμ� 0.1 mol��L-1 NaOH��Һ���õ���ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ��ʾ����NH4HSO4��Һ�еμ�NaOH��Һ��a��Ĺ����У�������Ӧ�����ӷ���ʽΪ__________________��
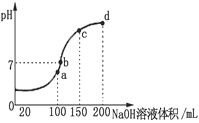
��4��Ũ�Ⱦ�Ϊ0.1 mol��L-1 NH4Al(SO4)2��Һ��NH4HSO4��Һ������______��Һc(NH4��)��
���𰸡� �� l.0��l0-3����l.0��l0-3-l.0��l0-11�� Al3+��NH4������ˮ��ƽ�⣺Al3++3H2O![]() Al(OH)3+3H+��NH4��+H2O
Al(OH)3+3H+��NH4��+H2O![]() NH3��H2O+H+�������¶���ˮ��̶�����c(H+)����pH��С H++OH-=== H2O NH4HSO4
NH3��H2O+H+�������¶���ˮ��̶�����c(H+)����pH��С H++OH-=== H2O NH4HSO4
����������1�����⿼�������غ�͵���غ㣬���������غ㣬�Լ�NH4Al(SO4)2���Ƴ�c(NH4��)��c(NH3��H2O)=c(Al3��)��c[Al(OH)3]�����ݵ���غ㣬c(NH4��)��3c(Al3��)��c(H��)=c(OH��)��2c((SO42��)�������2c(SO42��)��c(NH4��)��3c(Al3��)=c(H��)��c(OH��)=10��3��10��11����2�����⿼��Ӱ������ˮ������أ�NH4Al(SO4)2��Һ�����ԣ�����Ϊ����NH4����H2O![]() NH3��H2O��H����Al3����3H2O
NH3��H2O��H����Al3����3H2O![]() Al(OH)3��3H��������ˮ�������ȷ�Ӧ�������¶ȣ��ٽ�ˮ�⣬c(H��)����pH��С����3�����⿼��ζ�ʵ�飬NH4HSO4�ĵ��뷽��ʽΪNH4HSO4=NH4����H����SO42����H�����OH������ǿ��NH4������a��ʱͨ��NaOH�����Ϊ100mL��ǡ���к�NH4HSO4�����H����������ӷ�Ӧ����ʽΪH����OH��=H2O����4�����⿼��Ӱ������ˮ������أ�Al3����H��������NH4����ˮ�⣬H������NH4��ˮ������ǿ����NH4HSO4��Һ��c(NH4��)��
Al(OH)3��3H��������ˮ�������ȷ�Ӧ�������¶ȣ��ٽ�ˮ�⣬c(H��)����pH��С����3�����⿼��ζ�ʵ�飬NH4HSO4�ĵ��뷽��ʽΪNH4HSO4=NH4����H����SO42����H�����OH������ǿ��NH4������a��ʱͨ��NaOH�����Ϊ100mL��ǡ���к�NH4HSO4�����H����������ӷ�Ӧ����ʽΪH����OH��=H2O����4�����⿼��Ӱ������ˮ������أ�Al3����H��������NH4����ˮ�⣬H������NH4��ˮ������ǿ����NH4HSO4��Һ��c(NH4��)��

 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�����Ŀ�����Ĺ̶�һֱ�ǿ�ѧ���о�����Ҫ���⣬�ϳɰ������˹��̵��Ƚϳ���ļ�������ԭ��Ϊ:N2(g)+3H2(g)![]() 2NH3(g) ��H
2NH3(g) ��H
��1����֪�ƻ�1mol���ۼ���Ҫ�����������ʾ
H-H | N-H | N-N | N��N |
435.5kJ | 390.8kJ | 163kJ | 945.8kJ |
���H=__________��
��2���ں��¡���ѹ�����У��������1:3����N2��H2���кϳɰ���Ӧ���ﵽƽ������������г��������������ﵽ��ƽ��ʱ��c(H2)��__________�����������С�������䡱����ͬ�������ں��¡�����������c(N2)/c(NH3)��________��
��3���ڲ�ͬ�¶ȡ�ѹǿ��ʹ����ͬ���������£���ʼʱN2��H2�ֱ�Ϊ0.1mol��0.3mol ʱ��ƽ�������а�������������գ���ͼ��ʾ��

�����У�p1��p2��p3�ɴ�С��˳����_______��ԭ����___________________��
������250�桢p1�����£���Ӧ�ﵽƽ��ʱ���������Ϊ1L����������ºϳɰ���ƽ�ⳣ��K=____(���������λС��)��
��4��H2NCOONH4�ǹ�ҵ�ɰ����ϳ����ص��м�����һ���¶��¡����������ܱ������з�����ӦH2NCOONH4(s)![]() 2NH3(g)+CO2(g),��˵���÷�Ӧ�ﵽƽ��״̬����_____������ţ�
2NH3(g)+CO2(g),��˵���÷�Ӧ�ﵽƽ��״̬����_____������ţ�
��ÿ����34g NH3��ͬʱ����44g CO2 �ڻ��������ܶȱ��ֲ���
��NH3������������ֲ��� �ܻ�������ƽ����Է����������� ��c(NH3):c(CO2)=2:1
��5����ѧ�ҷ��֣�N2��H2��ɵ�ԭ��غϳɰ��빤ҵ�ϳɰ���Ⱦ���Ч�ʸߣ������״ﵽ���ŵ㡣��װ����ͼ��ʾ��д����ԭ��صĵ缫��Ӧ��________________��_____________����N2�����ڿ��������缫B��A��ͨ��2molH+ʱ��������Ҫ����¿��������Ϊ_________�����������λС������




