��Ŀ����
10�������й�˵��������ǣ�������| A�� | ��֪�Ȼ��ܼ���ˮ�������ֲ�ͬ��ɫ�����£����¹���ѧ�ҷ����������Ȼ��ܵı�ɫˮ�࣬�ݴ��Ʋ������ɫˮ��ʷۺ�ɫ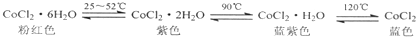 | |
| B�� | ��֪���������ɫ�����ظ��������ɫ�����Ӽ��������ƽ�⣺2CrO42-+2H+?Cr2O72-+H2O������ˮϡ�ͣ�����Һ����������Ũ�Ⱦ����� | |
| C�� | ����Ӧ2A��g��+B��s��?C��g��+3D��g��ֻ�и����²����Է����У���÷�Ӧ�ġ�H��0 | |
| D�� | ����Ӧ���Ũ�ȣ���ʹ��λ����ڻ������Ŀ���࣬��Ӧ���ʼӿ� |
���� A������ʱ�¶���Խϵͣ�ʪ�ȴ�ƽ�������ƶ���
B����ˮϡ�ͣ�ƽ�������ƶ���Cr2O72-���ʵ�������
C������ӦΪ�������̣���G=��H-T��S��0�Է����У��ݴ��жϣ�
D������Ӧ��Ũ�ȣ������Ũ������Ӧ��������
��� �⣺A������ʱ�¶���Խϵͣ�ʪ�ȴ�ƽ�������ƶ�����ҪΪCoCl2•6H2O���ʷۺ�ɫ����A��ȷ��
B����ˮϡ�ͣ���Һ�и�����Ũ�ȶ����ͣ���ƽ�������ƶ���Cr2O72-���ʵ�������B����
C������ӦΪ�������̣��ڸ������Է����У�������¡�G=��H-T��S��0�������ƶϡ�H��0����C��ȷ��
D������Ӧ��Ũ�ȣ���λ����ڻ���ӵ���Ŀ������Ч��ײ�Ĵ������࣬��Ӧ��������D��ȷ��
��ѡB��
���� ���⿼��ƽ���ƶ�Ӱ�����ء���Ӧ���з�����ӵȣ��ѶȲ���Bѡ��Ϊ�״��㣬����Ũ�������ʵ������������Ը���ƽ�ⳣ������ƽ���ƶ�����

��ϰ��ϵ�д�
 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�
�����Ŀ
20����NAΪ�����ӵ�������ֵ������������ȷ���ǣ�������
| A�� | 30g�������辧���к���Si-O����ĿΪ2NA | |
| B�� | ��״���£�2.24L CHCl3��ԭ������Ϊ0.1NA | |
| C�� | һ�������£���1molN2��3molH2��Ϸ�Ӧ������NH3���ӵ���ĿΪ2NA | |
| D�� | �����£�0.1mol/LNH4NO3��ҺNO${\;}_{3}^{-}$����ĿΪ0.1NA |
1����NA��ʾ�����ӵ�����������˵����ȷ���ǣ�������
| A�� | 23g��������������ַ�Ӧ��ת�Ƶĵ��Ӹ���ΪNA | |
| B�� | ��1molFeCl3ˮ���Ƴɽ��壬���ý��������ΪNA | |
| C�� | 1mol Na2O2��ˮ��ȫ��Ӧʱת�Ƶ�����Ϊ2NA | |
| D�� | NA��һ����̼���Ӻ�0.5 mol�����������Ϊ7��4 |
18�������£����и��������У�Y������X��Ӧ��������Z��Ӧ���ǣ�������
| ѡ�� | X | Y | Z |
| �� | N2 | Mg | CO2 |
| �� | KOH��Һ | SiO2 | ����� |
| �� | O2 | N2 | H2 |
| �� | ϡ���� | Fe | FeCl3��Һ |
| A�� | �٢� | B�� | �٢� | C�� | �ڢ� | D�� | �ڢ� |
5������˵���в���ȷ���ǣ�������
| A�� | ���ۻ�������һ�����������Ӽ������ӻ������п��ܺ��й��ۼ� | |
| B�� | ����صĻ�ѧ��Ӧԭ����������ԭ��Ӧ | |
| C�� | һ�������£���1 mol N2��3 mol H2�����ܱ������г�ַ�Ӧ������2 mol NH3 | |
| D�� | ��������ͬ��������Ҳ��ͬ������������������һ�ַ��Ӻ�һ������ |
15����NAΪ�����ӵ�������ֵ������˵����ȷ���ǣ�������
| A�� | 42g��ϩ�ͱ�ϩ�Ļ��������̼ԭ����Ŀһ��Ϊ3NA�� | |
| B�� | �ö��Ե缫���CuSO4��Һ���������0.1molCu��OH��2��ʹ��Һ��ԭ������ʱ������״����1.12L������ | |
| C�� | ����һ�����ʵ���Ũ�ȵ�NaOH��Һ����NaOH�������ձ��г���ܽ⣬��Ѹ��ת�Ƶ�����ƿ�ж��� | |
| D�� | 30g��NO��O2��ַ�Ӧ�����ɵ����������Ϊ1NA�� |
2��Ī���γ���������ԭ�ζ����Ļ����ʣ������йظ�����Һ�ıȽϣ�����ȷ���ǣ�������
| A�� | c��SO42-����c��NH4+����c��Fe2+����c��H+����c��OH-�� | B�� | c��SO42-��=c��NH4+����c��Fe2+����c��H+����c��OH-�� | ||
| C�� | 2c��SO42-��+c��OH-��=c��NH4+��+2c��Fe2+��+c��H+�� | D�� | c��NH4+��+c��NH3•H2O��=2c��Fe2+��+2c��SO42-�� |
19��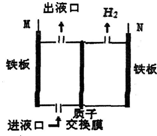 ��ͼ��ʾ��ij�������õ�ⷨ����������Cr2O72-�����Է�ˮ�����У��������������������������������Һ�е�Cr2O72-ȫ����ΪCr3+������˵������ȷ���ǣ�������
��ͼ��ʾ��ij�������õ�ⷨ����������Cr2O72-�����Է�ˮ�����У��������������������������������Һ�е�Cr2O72-ȫ����ΪCr3+������˵������ȷ���ǣ�������
 ��ͼ��ʾ��ij�������õ�ⷨ����������Cr2O72-�����Է�ˮ�����У��������������������������������Һ�е�Cr2O72-ȫ����ΪCr3+������˵������ȷ���ǣ�������
��ͼ��ʾ��ij�������õ�ⷨ����������Cr2O72-�����Է�ˮ�����У��������������������������������Һ�е�Cr2O72-ȫ����ΪCr3+������˵������ȷ���ǣ�������| A�� | M���Դ���������� | B�� | ��Һ���ų�����Һ�л�����Fe3+ | ||
| C�� | N���缫��ӦΪ2H++2e-�TH2�� | D�� | ������������ҺpH���� |
20�����������У�������ǣ�������
| A�� | ��ϩ����ʹ���Ը��������Һ��ɫ | |
| B�� | ������ʹ���Ը��������Һ��ɫ | |
| C�� | �Ҵ����Ժͽ����Ʒ�Ӧ�ų����� | |
| D�� | ����������Ҵ���һ�������·���������Ӧ |