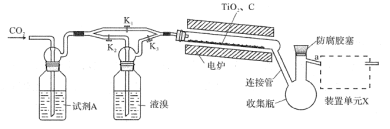
��Ŀ����
����Ŀ���ϳ���(CO��H2)��һ����Ҫ�Ļ���ԭ�������ϳ�����ȡ�ж��ַ�������ú����������Ȼ�����������ȡ��ش��������⣺
I.�ϳ�������ȡ
��1��ú��������ȡ�ϳ�����
��֪����H2O(g)=H2O(l) ��H=-44kJ/mol��
�ڲ������ʵ�ȼ���ȣ�
![]()
��ӦC(s)+H2O(g)![]() CO(g)+H2(g)�ġ�H=___kJ/mol��
CO(g)+H2(g)�ġ�H=___kJ/mol��
��2����Ȼ������������ȡ�ϳ�����
�����O2(g)��H2O(g)��CO2(g)���������CH4(g)����ʹ�Ƶõĺϳ�����CO��H2�����ʵ���֮��Ϊ1�U2����ԭ�������H2O(g)��CO2(g)�����ʵ���֮��Ϊ__��
��.���úϳ����ϳ��Ҵ�
��һ�������£����ݻ�Ϊ2L�ĺ����ܱ�������Ͷ��2molCO��4molH2��������Ӧ��2CO(g)+4H2(g)![]() CH3CH2OH(g)+H2O(g)��
CH3CH2OH(g)+H2O(g)��
��1��д���÷�Ӧ��ƽ�ⳣ������ʽ__��
��2�������������Ϊ�жϷ�Ӧ��ϵ�ﵽƽ��ı�־��__(�����)��
A.ѹǿ���ٱ仯 B.ƽ��Ħ���������ٱ仯 C.�ܶȲ��ٱ仯
��3����Ӧ��ʼѹǿ��Ϊp1��ƽ����Ϊp2��ƽ��ʱH2��ת����Ϊ__��(�ú�p1��p2�Ĵ���ʽ��ʾ)
��.�ϳ��Ҵ�������ѡ��
Ϊ̽���ϳ�����ȡ�Ҵ�������������ij�����ŶӶԲ�ͬ�¶ȡ���ͬRh���������Ĵ�����CO������ǿ�Ƚ������о���ʵ��������ͼ��CO�ķ����������ָCO��δ�Ҵ��������������ָCO�Ѿ��Ҵ�����
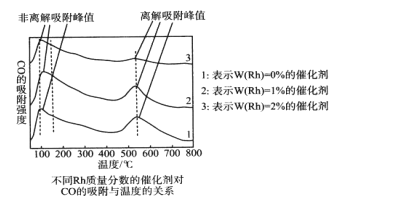
��1�����ͼ��ӵ������������������¶ȶ�CO����ǿ�ȵ�Ӱ��__���Լ�������CO����ǿ�ȵ�Ӱ��__��
��2����Rh���������ϳ�����ȡ�Ҵ��������¶���__��
���𰸡�+131.3 2��1 ![]() AB
AB ![]() ��100% �ڵ��������¶����ߣ���ͬ������CO�ķ��������ǿ�Ⱦ������ڸ��������¶����ߣ���ͬ������CO���������ǿ�Ⱦ���С ��ͬ�¶��£�������Rh��������Խ�ߣ�CO������ǿ��Խ�� 550��
��100% �ڵ��������¶����ߣ���ͬ������CO�ķ��������ǿ�Ⱦ������ڸ��������¶����ߣ���ͬ������CO���������ǿ�Ⱦ���С ��ͬ�¶��£�������Rh��������Խ�ߣ�CO������ǿ��Խ�� 550��
��������
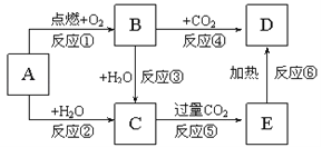
I.(1)��д��C��CO��H2ȼ�յ��Ȼ�ѧ��Ӧ����ʽ��Ȼ����ݸ�˹���ɵõ������
(2)�ֱ���д��CH4��O2��CH4��H2O��CH4��CO2��Ӧ�ķ���ʽ�����ݷ�Ӧ����ʽ���ص㣬���з����жϣ�
II.(1)���ݻ�ѧƽ�ⳣ���Ķ�����з�����
(2)���ݻ�ѧƽ��״̬�Ķ�����з�����
I.(1)C(s)��O2(g)��CO2(g) ��H=��393.5kJ��mol��1 �٣�
CO(g)��![]() O2(g)=CO2(g) ��H=��283.0kJ��mol��1 ��,
O2(g)=CO2(g) ��H=��283.0kJ��mol��1 ��,
H2(g)��![]() O2(g)=H2O(l) ��H=��285.8kJ��mol��1 �ۣ�
O2(g)=H2O(l) ��H=��285.8kJ��mol��1 �ۣ�
H2O(g)=H2O(l) ��H=��44kJ��mol��1 �ܣ����ݸ�˹���ɣ��٣��ܣ��ۣ��ڣ��ó���H=��131.3kJ��mol��1��
(2)�ֱ����ķ���ʽΪCH4��![]() O2=CO��2H2��CH4��CO2=2CO��2H2��CH4��H2O=CO��3H2��Ҫ��ϳ�����CO��H2�����ʵ���֮��Ϊ1��2��O2����������ֵ��ֻ����CO2��H2O��Ӧ��CO�����ʵ�����H2�����ʵ���֮��Ϊ1��2���ɣ���CO2Ϊamol��������n(CO)=2amol��n(H2)=2amol����H2OΪbmol��������n(CO)=bmol��n(H2)=3bmol����(2a��b)��(2a��3b)=1��2�����a��b=1��2��
O2=CO��2H2��CH4��CO2=2CO��2H2��CH4��H2O=CO��3H2��Ҫ��ϳ�����CO��H2�����ʵ���֮��Ϊ1��2��O2����������ֵ��ֻ����CO2��H2O��Ӧ��CO�����ʵ�����H2�����ʵ���֮��Ϊ1��2���ɣ���CO2Ϊamol��������n(CO)=2amol��n(H2)=2amol����H2OΪbmol��������n(CO)=bmol��n(H2)=3bmol����(2a��b)��(2a��3b)=1��2�����a��b=1��2��
II.(1�����ݻ�ѧƽ�ⳣ���Ķ��壬K=![]() ;
;
(2)A. ��Ϊ��Ӧǰ������ϵ��֮�Ͳ���ȣ���˵�ѹǿ���ٸı䣬˵����Ӧ�ﵽƽ�⣬��A�������⣻
B. ��ֶ������壬�����������ֲ��䣬�÷�ӦΪ�������ʵ������ٵķ�Ӧ������Ħ�������Ķ��壬��˵�Ħ���������ٸı䣬˵����Ӧ�ﵽƽ�⣬��B�������⣻
C. ��������������ֲ��䣬����Ϊ���ݣ�����ܶȲ��䣬����˵����Ӧ�ﵽƽ�⣬��C���������⣻
(3)��ͬ�����£�ѹǿ֮�ȵ������ʵ��������ﵽƽ������������ʵ���Ϊ![]() mol��
mol��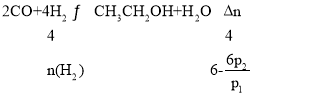 �����n(H2)=
�����n(H2)= ![]() mol����H2��ת����Ϊ
mol����H2��ת����Ϊ![]() =
=![]() ��100%��
��100%��
III.(1)����ͼ���ڵ��������¶����ߣ���ͬ������CO�ķ��������ǿ�Ⱦ������ڸ��������¶����ߣ���ͬ������CO���������ǿ�Ⱦ���С����ͬ�¶��£�������Rh��������Խ�ߣ�CO������ǿ��Խ��
(2)����ͼ���������¶�Ϊ550�档

����Ŀ���±�ΪԪ�����ڱ���һ���֣������Ԫ���������ڱ��е�λ�ã��û�ѧ����ش��������⣺
�� ���� | IA | 0 | ||||||
1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | ||||
��1������ԭ�ӽṹʾ��ͼΪ_________��
��2��������̬�⻯����ӵĽṹʽΪ___________������������̬�⻯����ȶ�����ȣ����н�������____ (�ø��⻯��Ļ�ѧʽ��ʾ)��
��3������������ۺ������������ǿ������˳����____���ѧʽ����
��4��������Ԫ�صĽ�����ǿ������Ϊ___________�����������С�����䡱����
��5���������������γɵļ����Ӱ뾶����_________�����������С�����䡱����
��6������������Ԫ�ؿ��γɼȺ����Ӽ��ֺ����ۼ��Ļ����д�����ĵ���ʽ��_____��