��Ŀ����
����Ŀ����ҵ�ϲ�����������ȥ��������(��Ҫ�ɷ�ΪFe2O3��FeO��SiO2��Al2O3����������������) ��ȡ��ˮ����������(FeSO4��7H2O) ���������£�
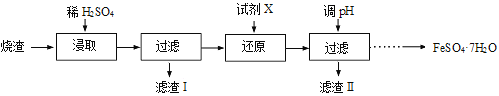
��1����ȡʱ����Һ�е�Fe2+�ױ������е�O2�����������ӷ���ʽΪ �������������ȡ���ʵĴ�ʩ�� ������ĸ����
A������������ B�����������Ũ�� C���ʵ������¶�
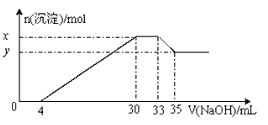
��2����ԭʱ���Լ�X����������ҺpH�ı仯��ͼ��ʾ�����Լ�X������ ������ĸ����
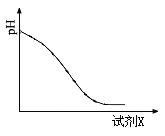
A��Fe�� B��SO2 C��NaI
��ԭ����ʱ����Һ�е���Ҫ�������� ��
��3����������Ҫ�ɷֵĻ�ѧʽΪ ���ɷ���������������Һ�õ���Ʒ�����еIJ����� �� ���ˡ�ϴ�ӡ����
���𰸡�
��1��4Fe2++O2+4H+=4Fe3++2H2O��AC��
��2��B��SO42-��
��3��Al(OH)3������Ũ������ȴ�ᾧ��
��������
������1��Fe2+�ױ������е�O2��������Fe3+�����ӷ���ʽΪ4Fe2++O2+4H+=4Fe3++2H2O��A�����������������˷�Ӧ��ĽӴ��������Ӧ���ʼӿ죬��ȷ��B�����������Ũ������Ӧ���ʼ���������C�������¶�����Ӧ���ʼӿ죬��ȷ����ѡAC���ʴ�Ϊ��4Fe2++O2+4H+=4Fe3++2H2O��AC��
��2��A��Fe����Fe3+��Ӧ����Fe2+����Һ������Ի������䣬����B��SO2��Fe3+��Ӧ�������ᣬ��Һ��������ǿ����ȷ��C��NaI��Fe3+��Ӧ����I2����Һ������Ի������䣬������ԭ����ʱ����Һ�е���Ҫ��������SO42-���ʴ�Ϊ��B��SO42-��
��3����һ�ι��˺���Һ�д���Fe3+��Al3+������X��Fe3+��ԭΪFe2+��Al3+������Һ�У�����pH��Ŀ���dz�ȥAl3+�������������Ҫ�ɷ�ΪAl(OH)3���������������Һ�õ���Ʒ�����еIJ���������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ��������ʴ�Ϊ:Al(OH)3������Ũ������ȴ�ᾧ��