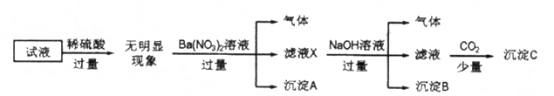
ƒøƒ⁄»ð
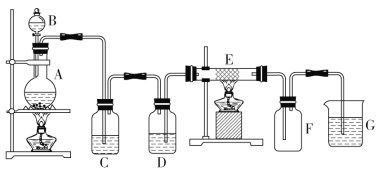
°æƒø°øƒ≥—ß…˙”˚”√œ¬¡–◊∞÷√÷∆»°¥øæªFeCl3πÃð£∆‰÷–A «Cl2µƒ∑¢…˙◊∞÷√£¨C°¢DŒ™∆¯ÃÂ檪Ø◊∞÷√£¨E”≤÷ ≤£¡ßπÐ÷–◊∞”–œ∏Ã˙ÀøÕ¯£¨FŒ™∏…‘Ôµƒø’π„ø⁄∆ø£¨…’±≠GŒ™Œ≤∆¯Œ¸ ’◊∞÷√°£
‘ªÿ¥£∫
(1)∏√◊∞÷√÷–”–“ª√˜œ‘¥ÌŒÛ «____________£®ÃÓ◊÷ƒ∏£©°£
(2)D÷–À˘◊∞ ‘º¡Œ™_____________£¨C◊∞÷√µƒ◊˜”√ «___________________°£
(3)A÷–∑¢…˙∑¥”¶µƒªØ—ß∑Ω≥Ã Ω «__________________£¨G÷–∑¢…˙∑¥”¶µƒ¿Î◊”∑Ω≥Ã Ω «________________°£
(4)»Ù”√100mL 12mol/L—ŒÀ·”Î◊„¡øMnO2≥‰∑÷∑¥”¶£¨…˙≥…µƒCl2ê˝(±Í◊º◊¥øˆ)________(ÃÓ°∞¥Û”⁄°±°∞–°”⁄°±ªÚ°∞µ»”⁄°±)6.72 L°£
(5)”√À˘÷∆»°µƒFeCl3πÃÃÂ÷∆≥…Fe(OH)3Ω∫㨫¯∑÷Ω∫ÃÂ∫ջГ∫≥£”√________£¨∑÷¿ÎΩ∫ÃÂ∫ջГ∫≥£”√µƒ∑Ω∑®Ω–_______________°£
°æ¥∞∏°ø◊∞÷√D ≈®H2SO4 ≥˝»•Cl2÷–µƒHCl∆¯Ã MnO2+4HCl(≈®)![]() MnCl2+Cl2°¸+2H2O Cl2+2OH-=Cl-+ClO-+H2O –°”⁄ ∂°¥Ô∂˚–ß”¶ …¯Œˆ
MnCl2+Cl2°¸+2H2O Cl2+2OH-=Cl-+ClO-+H2O –°”⁄ ∂°¥Ô∂˚–ß”¶ …¯Œˆ
°æΩ‚Œˆ°ø
‘⁄◊∞÷√A÷–”√MnO2°¢HCl(≈®)ªÏ∫œº”»»÷∆»°Cl2£¨◊∞÷√C≥˝»•‘”÷ HCl£¨◊∞÷√D≥˝»•Cl2÷–µƒÀÆ’Ù∆¯£¨‘⁄◊∞÷√E÷–Fe”ÎCl2∑¥”¶≤˙…˙FeCl3£¨◊∞÷√F «∞≤»´∆ø£¨∑¿÷πµπŒ¸œ÷œÛµƒ∑¢…˙£¨◊∞÷√G «Œ≤∆¯¥¶¿Ì◊∞÷√£¨”√”⁄≥˝»•Œ¥∑¥”¶µƒCl2£¨∏˘æðΩ∫ÃÂŒ¢¡£µƒ¥Û–°∑÷Œˆ≈–∂œŒÔ÷ µƒº¯±∫Õ÷¥ø°£
(1)‘⁄◊∞÷√A÷–÷∆»°Cl2£¨∏√∑¥”¶–Ë“™º”»»£¨HClæþ”–ª”∑¢–‘£¨À˘“‘÷∆»°µƒCl2÷–∫¨”–‘”÷ HCl°¢H2O’Ù∆¯£¨◊∞÷√C≥˝»•HCl£¨◊∞÷√D≥˝»•ÀÆ’Ù∆¯£¨µº∆¯πÐ «≥§Ω¯∂Ã≥ˆ£ª◊∞÷√Õº÷–µº∆¯πÐ «∂ÃΩ¯≥§≥ˆ£¨À˘“‘∏√◊∞÷√÷–”–“ª√˜œ‘¥ÌŒÛ « «◊∞÷√D£ª
(2)D÷–À˘◊∞ ‘º¡Œ™≈®¡ÚÀ·£¨◊˜”√ «∏…‘ÔCl2£¨C◊∞÷√µƒ◊˜”√ «≥˝»•Cl2÷–µƒHCl£ª
(3)‘⁄A÷–MnO2°¢HCl(≈®)ªÏ∫œº”»»÷∆»°Cl2£¨∑¢…˙∑¥”¶µƒªØ—ß∑Ω≥Ã Ω «MnO2+4HCl(≈®)![]() MnCl2+Cl2°¸+2H2O£¨G÷–Cl2”ÎNaOH»Ð“∫∑¥”¶≤˙…˙NaCl°¢NaClO°¢H2O£¨∑¢…˙∑¥”¶µƒ¿Î◊”∑Ω≥Ã Ω «Cl2+2OH-=Cl-+ClO-+H2O£ª
MnCl2+Cl2°¸+2H2O£¨G÷–Cl2”ÎNaOH»Ð“∫∑¥”¶≤˙…˙NaCl°¢NaClO°¢H2O£¨∑¢…˙∑¥”¶µƒ¿Î◊”∑Ω≥Ã Ω «Cl2+2OH-=Cl-+ClO-+H2O£ª
(4)»Ù”√100mL 12mol/L—ŒÀ·”Î◊„¡øMnO2≥‰∑÷∑¥”¶£¨n(HCl)=12mol/L°¡0.1L=1.2mol£¨»ÙHClÕÍ»´∑¥”¶£¨‘Ú∏˘æð∑¥”¶≤˙…˙Cl2µƒŒÔ÷ µƒ¡øn(Cl2)=1.2mol°¬4=0.3mol£¨∆‰‘⁄±Í◊º◊¥øˆÃª˝Œ™6.72L£¨µ´ µ÷ …œ£¨ÀÊ◊≈∑¥”¶µƒΩ¯––£¨≈®—ŒÀ·±‰Œ™œ°—ŒÀ·£¨∑¥”¶æÕ≤ª‘Ÿ∑¢…˙£¨“Ú¥À∑¥”¶≤˙…˙µƒCl2‘⁄±Í◊º◊¥øˆœ¬Ãª˝–°”⁄6.72L£ª
(5)”√À˘÷∆»°µƒFeCl3πÃÃÂ÷∆≥…Fe(OH)3Ω∫㨫¯∑÷Ω∫ÃÂ∫ջГ∫≥£”√∂°¥Ô∂˚–ß”¶£¨”…”⁄Ω∫ÃÂŒ¢¡£≤ªƒÐÕ®π˝∞ÎÕ∏ƒ§£¨»Ð“∫µƒŒ¢¡£ƒÐπªÕ®π˝∞ÎÕ∏ƒ§£¨À˘“‘∑÷¿ÎΩ∫ÃÂ∫ջГ∫≥£”√µƒ∑Ω∑®Ω–…¯Œˆ°£

 ‘ƒ∂¡øÏ≥µœµ¡–¥∞∏
‘ƒ∂¡øÏ≥µœµ¡–¥∞∏