题目内容
【题目】(1) 在微生物作用的条件下,NH![]() 经过两步反应被氧化成NO
经过两步反应被氧化成NO![]() 。两步反应的能量变化示意图如下:
。两步反应的能量变化示意图如下:

① 第一步反应是________(填“放热”或“吸热”)反应,判断依据是________________。
② 1 mol NH![]() (aq)全部氧化成NO
(aq)全部氧化成NO![]() (aq)的热化学方程式是_______________________。
(aq)的热化学方程式是_______________________。
(2) 已知红磷比白磷稳定,则反应P4(白磷,s)+5O2(g)===2P2O5(s) ΔH1;4P(红磷,s)+5O2(g)===2P2O5(s) ΔH2;ΔH1和ΔH2的关系是ΔH1________ΔH2 (填“>”“<”或“=”)。
(3) 在298 K、101 kPa时,已知:2H2O(g)===O2(g)+2H2(g) ΔH1
Cl2(g)+H2(g)===2HCl(g) ΔH2
2Cl2(g)+2H2O(g)===4HCl(g)+O2(g) ΔH3
则ΔH3与ΔH1和ΔH2之间的关系正确的是________。
A.ΔH3=ΔH1+2ΔH2 B.ΔH3=ΔH1+ΔH2
C.ΔH3=ΔH1-2ΔH2 D.ΔH3=ΔH1-ΔH2
(4) 已知:
2CO(g)+O2(g)===2CO2(g) ΔH=-566 kJ·mol-1 ①
Na2O2(s)+CO2(g)===Na2CO3(s)+![]() O2(g) ΔH=-226 kJ·mol-1 ②
O2(g) ΔH=-226 kJ·mol-1 ②
则CO(g)与Na2O2(s)反应放出509 kJ热量时,电子转移数目为________________。
(5) 已知H2(g)+Br2(l)===2HBr(g) ΔH=-72 kJ·mol-1,蒸发1 mol Br2(l)需要吸收的能量为30 kJ,其他相关数据如下表:
物质 | H2(g) | Br2(g) | HBr(g) |
1 mol分子中的化学键断 裂时需要吸收的能量(kJ) | 436 | 200 | a |
则表中a=________。
【答案】 放热 ΔH<0(或反应物的总能量大于生成物的总能量) NH![]() (aq)+2O2(g)===NO
(aq)+2O2(g)===NO![]() (aq)+2H+(aq)+H2O(l) ΔH=-346 kJ·mol-1 < A 1.204×1024 (或2NA) 369
(aq)+2H+(aq)+H2O(l) ΔH=-346 kJ·mol-1 < A 1.204×1024 (或2NA) 369
【解析】(1)①由图可知,焓变小于0,即反应物的总能量大于生成物的总能量,所以反应为放热反应,故答案为:放热;反应物的总能量大于生成物的总能量;
②第一步的热化学方程式为NH4+(aq)+1.5O2(g)═NO2-(aq)+2H+(aq)+H2O(l),△H=-273kJ/mol,第二步的热化学方程式为:NO2-(aq)+0.5O2(g)═NO3-(aq),△H=-73kJ/mol,根据盖斯定律则NH4+(aq)+2O2(g)═2H+(aq)+H2O(l)+NO3-(aq),△H=-346 kJ/mol,故答案为:NH4+(aq)+2O2(g)═2H+(aq)+H2O(l)+NO3-(aq),△H=-346 kJ/mol;
(2)①4P(白磷,s)+5O2(g)=2P2O5(s)△H1,②P4(红磷,s)+5O2(g)=2P2O5(s)△H2,①-②得到反应:4P(红磷,s)=P4(白磷,s)△H=△H2-△H2,红磷比白磷稳定,说明红磷的能量低于白磷,该反应是吸热反应,即△H2-△H1>0,即△H2>△H1,故答案为:<;(3)①2H2O(g)═O2(g)+2H2(g)△H1;②Cl2(g)+H2(g)═2HCl(g)△H2;③2Cl2(g)+2H2O(g)=4HCl(g)+O2(g)△H3;则反应③=2×②+①,由盖斯定律可知,△H3=△H1+2△H2,故答案为:A;
(4) 已知:①2CO(g)+O2(g)=2CO2(g);△H=-566kJ/mol,②Na2O2(s)+CO2(g)=Na2CO3(s)+ ![]() O2(g);△H=-226kJ/mol,根据盖斯定律,②×2+①可得:2CO(g)+2Na2O2(s)=2 Na2CO3(s) △H=-1018kJ/mol,CO(g)与Na2O2(s)反应放出509kJ热量时,参加反应CO为2mol×
O2(g);△H=-226kJ/mol,根据盖斯定律,②×2+①可得:2CO(g)+2Na2O2(s)=2 Na2CO3(s) △H=-1018kJ/mol,CO(g)与Na2O2(s)反应放出509kJ热量时,参加反应CO为2mol×![]() =1mol,反应的电子转移数为2mol,即电子转移数为1.204×1024,故答案为:1.204×1024;
=1mol,反应的电子转移数为2mol,即电子转移数为1.204×1024,故答案为:1.204×1024;
(5)H2(g)+Br2(l)=2HBr(g)△H=-72kJ/mol,蒸发1 mol Br2(l)需要吸收的能量为30 kJ,则H2(g)+Br2(g)=2HBr(g)△H=-102kJ/mol,△H=反应物键能-生成物键能,则有-102=436+200-2a,a=369,故答案为:369。

【题目】回答下列以下有关酶的问题:
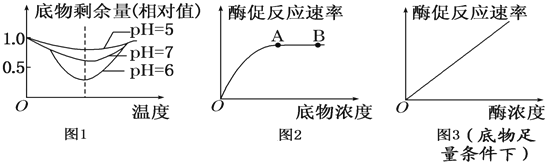
(1)由图1曲线可知:当pH从5上升到7,酶活性的变化过程是 ;从图示曲线还可以得出的结论是 .
(2)图2和图3是底物浓度和酶浓度对酶促反应的影响曲线,请分析回答:
图2中A点后酶促反应的速率不再增加,其限制性因素主要是 .从图3可以得出的结论是:在底物足量的条件下, .
(3)以下是一个有关酶的实验,根据表格内容分析回答下列问题:
操作步骤 | 操作方法 | 试管A | 试管B | 试管C |
1 | 加淀粉溶液 | 2mL | 2mL | 2mL |
2 | 加淀粉酶溶液 | 1mL | 1mL | 1mL |
3 | 温度处理 | 60℃ | 100℃ | 0℃ |
4 | 加碘液 | 2滴 | 2滴 | 2滴 |
①表中为探究 的实验.
②请指出实验操作步骤存在的问题 .
③该实验把温度处理改为pH分别为5、7、9能否用于探究pH对酶活性的影响? 说明理由 .

