��Ŀ����
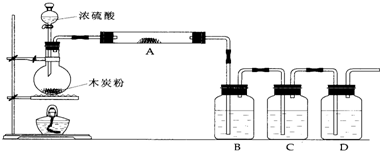
ijʵ��С���ͬѧ����ͭ��Ũ���ᷴӦ��ʵ��ʱ�����ַ�Ӧ�������Թܵײ��а�ɫ���岢������������ɫ���ʣ���ȥ�Թ��е�Ũ���ᣬ��ʣ����壨������Ũ���ᣩ����ʢ��ˮ���ձ��У�����������ҺΪ��ɫ��ͬʱ���к�ɫ���壮
��1��������ʵ���֪��ԭ�Թܵײ��İ�ɫ������______��
��2��С��ͬѧ��ΪCuO���ܲ�����Ũ���Ტ�²��ɫ����ΪCuO��С����������ʵ����������С��IJ²⣬С����������______��
��3��Ϊ̽����ɫ�������ɣ�С���Ա���������֪��ɫ���������CuS��Cu2S�����ǵĻ���ͬʱ��֪CuS��Cu2S�ֱ��ܱ�Ũ��������ΪCu2+��
��CuS��Cu2S�ֱ����ڿ�������������CuO��SO2��
��д��CuS��������Ũ����Ļ�ѧ����ʽ��______��
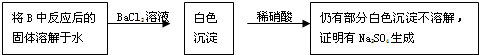
�ڽ��ձ��к�ɫ������˲�ϴ�Ӹɾ�������Ũ���ᣬ�������ܽ���ټ�������BaCl2��Һ����ʵ������У���˵����ɫ�����к�Cu��S����Ԫ�ص�ʵ��������______��
�������Ǹ�ʵ��С���Ա��Ҫ��һ��̽����ɫ���嵽����CuS��Cu2S�������ǵĻ���֣�Ӧ������ʵ�飿���Ҫ������______��
��1��������ʵ���֪��ԭ�Թܵײ��İ�ɫ������______��
��2��С��ͬѧ��ΪCuO���ܲ�����Ũ���Ტ�²��ɫ����ΪCuO��С����������ʵ����������С��IJ²⣬С����������______��
��3��Ϊ̽����ɫ�������ɣ�С���Ա���������֪��ɫ���������CuS��Cu2S�����ǵĻ���ͬʱ��֪CuS��Cu2S�ֱ��ܱ�Ũ��������ΪCu2+��
| SO | 2-4 |
��д��CuS��������Ũ����Ļ�ѧ����ʽ��______��
�ڽ��ձ��к�ɫ������˲�ϴ�Ӹɾ�������Ũ���ᣬ�������ܽ���ټ�������BaCl2��Һ����ʵ������У���˵����ɫ�����к�Cu��S����Ԫ�ص�ʵ��������______��
�������Ǹ�ʵ��С���Ա��Ҫ��һ��̽����ɫ���嵽����CuS��Cu2S�������ǵĻ���֣�Ӧ������ʵ�飿���Ҫ������______��
��1��Cu��ŨH2SO4������ӦΪCu+2H2SO4��Ũ��
CuSO4+SO2+2H2O������ŨH2SO4������Ũ���������ˮ�ԣ������ð�ɫ����ΪCuSO4���ʴ�Ϊ��CuSO4��
��2������Ũ���������CuO����ϡ���ᣬ��ɫ���岻����ΪCuO���ʴ�Ϊ���ձ��к���ϡ���ᣬ����������ͭ���ܽ���ϡ�����У�
��3����CuS��������Ũ����Ļ�ѧ����ʽΪ��CuS+8HNO3=CuSO4+8NO2��+4H2O���ʴ�Ϊ��CuS+8HNO3=CuSO4+8NO2��+4H2O��
�ڽ��ձ��к�ɫ������˲�ϴ�Ӹɾ�������Ũ���ᣬ�������ܽ���ټ�������BaCl2��Һ����ʵ������У���˵����ɫ�����к�Cu��S����Ԫ�ص�ʵ�������ǣ�������Ũ���ᣬ��Һ����ɫ��˵����Cu2+������BaCl2��Һ�����˲���������ij���BaSO4��ԭ��ɫ�����к�SԪ�أ���Cu��S��Ԫ�أ�����ΪCuSҲ����ΪCu2S��
�ʴ�Ϊ��������ҺΪ��ɫ�������Ȼ�����Һ���а�ɫ�������ɣ�
��Ҫ��һ��̽����ɫ���嵽����CuS��Cu2S�������ǵĻ����������ý���ɫ�����ڿ����г������Ȼ��ȽϷ�Ӧǰ����������ı仯������2CuS+3O2=2CuO+SO2��Cu2S+2O2=2CuO+SO2������Ӧ�������������ΪCu2S��������������СΪCuS��
�ʴ�Ϊ������ɫ�����ڿ����г������Ȼ��ȽϷ�Ӧǰ����������ı仯������������ΪCu2S����������СΪCuS��
| ||
��2������Ũ���������CuO����ϡ���ᣬ��ɫ���岻����ΪCuO���ʴ�Ϊ���ձ��к���ϡ���ᣬ����������ͭ���ܽ���ϡ�����У�
��3����CuS��������Ũ����Ļ�ѧ����ʽΪ��CuS+8HNO3=CuSO4+8NO2��+4H2O���ʴ�Ϊ��CuS+8HNO3=CuSO4+8NO2��+4H2O��
�ڽ��ձ��к�ɫ������˲�ϴ�Ӹɾ�������Ũ���ᣬ�������ܽ���ټ�������BaCl2��Һ����ʵ������У���˵����ɫ�����к�Cu��S����Ԫ�ص�ʵ�������ǣ�������Ũ���ᣬ��Һ����ɫ��˵����Cu2+������BaCl2��Һ�����˲���������ij���BaSO4��ԭ��ɫ�����к�SԪ�أ���Cu��S��Ԫ�أ�����ΪCuSҲ����ΪCu2S��
�ʴ�Ϊ��������ҺΪ��ɫ�������Ȼ�����Һ���а�ɫ�������ɣ�
��Ҫ��һ��̽����ɫ���嵽����CuS��Cu2S�������ǵĻ����������ý���ɫ�����ڿ����г������Ȼ��ȽϷ�Ӧǰ����������ı仯������2CuS+3O2=2CuO+SO2��Cu2S+2O2=2CuO+SO2������Ӧ�������������ΪCu2S��������������СΪCuS��
�ʴ�Ϊ������ɫ�����ڿ����г������Ȼ��ȽϷ�Ӧǰ����������ı仯������������ΪCu2S����������СΪCuS��

��ϰ��ϵ�д�
�����Ŀ