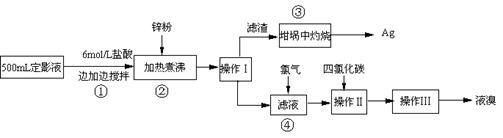
��Ŀ����
��֪��ˮ�ĵ���������ĵ������ͬ��ͬŨ������ȣ�����һ���������Ȼ����Һ�ʼ��ԣ�����������Mg��OH��2����Һ�У����������ı����Ȼ����Һ��������ȫ�ܽ⣮
��ͬѧ�Ľ����ǣ�Mg��OH��2���̣�?Mg2++2OH-����
NH4++H2O?NH3?H2O+H+����H++OH-=H2O����
����NH4+ˮ�������ԣ�H+��OH-��Ӧ����ˮ�����·�Ӧ��ƽ�����ƣ������ܽ⣻
��ͬѧ�Ľ����ǣ�Mg��OH��2���̣�?Mg2++2OH-����NH4++OH-?NH3?H2O����
����NH4Cl�������NH4+��Mg��OH��2�������OH-��ϣ����������ĵ����NH3?H2O�����·�Ӧ�ٵ�ƽ�����ƣ�Mg��OH��2�����ܽ⣮
��1����ͬѧ���ܿ϶���λͬѧ�Ľ��ͺ���������ѡ�����е�һ���Լ�����֤���ס�����λͬѧ�Ľ���ֻ��һ����ȷ����ѡ�õ��Լ���______����д��ţ���
A��NH4NO3B��CH3COONH4C��Na2CO3D��NH3?H2O
��2������˵����ͬѧ������ѡ�������______
��3����ͬѧ����ѡ�Լ�����Mg��OH��2����Һ�У�Mg��OH��2�ܽ⣻�ɴ���֪��������λͬѧ�Ľ�������______����ס����ҡ��������NH4Cl������ҺʹMg��OH��2�����ܽ�����ӷ���ʽ______��
��ͬѧ�Ľ����ǣ�Mg��OH��2���̣�?Mg2++2OH-����
NH4++H2O?NH3?H2O+H+����H++OH-=H2O����
����NH4+ˮ�������ԣ�H+��OH-��Ӧ����ˮ�����·�Ӧ��ƽ�����ƣ������ܽ⣻
��ͬѧ�Ľ����ǣ�Mg��OH��2���̣�?Mg2++2OH-����NH4++OH-?NH3?H2O����
����NH4Cl�������NH4+��Mg��OH��2�������OH-��ϣ����������ĵ����NH3?H2O�����·�Ӧ�ٵ�ƽ�����ƣ�Mg��OH��2�����ܽ⣮
��1����ͬѧ���ܿ϶���λͬѧ�Ľ��ͺ���������ѡ�����е�һ���Լ�����֤���ס�����λͬѧ�Ľ���ֻ��һ����ȷ����ѡ�õ��Լ���______����д��ţ���
A��NH4NO3B��CH3COONH4C��Na2CO3D��NH3?H2O
��2������˵����ͬѧ������ѡ�������______
��3����ͬѧ����ѡ�Լ�����Mg��OH��2����Һ�У�Mg��OH��2�ܽ⣻�ɴ���֪��������λͬѧ�Ľ�������______����ס����ҡ��������NH4Cl������ҺʹMg��OH��2�����ܽ�����ӷ���ʽ______��
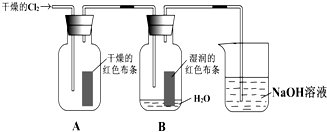
��1������狀��Ȼ�����ƣ�ֻ��笠����ӵ�ˮ�⣬��̼���ƺͰ�ˮ��Һ���ʼ��ԣ�ע��B���ϣ�
�ʴ�Ϊ��B��
��2���������Һ�����ԣ���֤���Ƿ�����笠�����ˮ������Ե�ԭ����������þ�ܽ⣬
�ʴ�Ϊ���������Һ�����ԣ�
��3������ѡ�Լ�����Mg��OH��2����Һ�У�Mg��OH��2�ܽ⣬��˵����ͬѧ�Ľ�����ȷ��
��NH4Cl������ҺʹMg��OH��2�����ܽ�����ӷ���ʽΪ2NH4++Mg��OH��2=2NH3?H2O+Mg2+��
�ʴ�Ϊ���Ȼ����Һ�ң�2NH4++Mg��OH��2=2NH3?H2O+Mg2+��
�ʴ�Ϊ��B��
��2���������Һ�����ԣ���֤���Ƿ�����笠�����ˮ������Ե�ԭ����������þ�ܽ⣬
�ʴ�Ϊ���������Һ�����ԣ�
��3������ѡ�Լ�����Mg��OH��2����Һ�У�Mg��OH��2�ܽ⣬��˵����ͬѧ�Ľ�����ȷ��
��NH4Cl������ҺʹMg��OH��2�����ܽ�����ӷ���ʽΪ2NH4++Mg��OH��2=2NH3?H2O+Mg2+��
�ʴ�Ϊ���Ȼ����Һ�ң�2NH4++Mg��OH��2=2NH3?H2O+Mg2+��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ