��Ŀ����
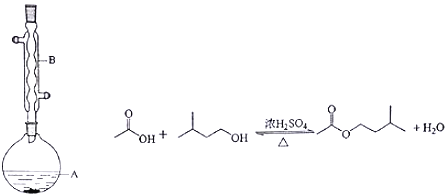
����Ŀ�������о����֣��ø�Ĥ��ⷨ������Ũ����ȩ��ˮ���й������̼���Ľϵ͵��ŵ㣬��ԭ����ʹ��ȩ�ֱ�����������������Ӧ��ת��Ϊ�Ҵ������ᣬ�ܷ�ӦΪ��2CH3CHO + H2O![]() CH3CH2OH + CH3COOH
CH3CH2OH + CH3COOH
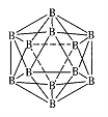
ʵ�����У���һ��Ũ�ȵ���ȩ��Na2SO4��ҺΪ�������Һ��ģ����ȩ��ˮ�Ĵ������̣���װ��ʾ��ͼ����ͼ��ʾ��
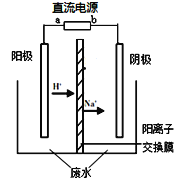
��1�����Լ���ȼ�ϵ��Ϊֱ����Դ����ȼ�ϵ����b��Ӧͨ�� ���ѧʽ�����塣
��2���������У��������ֱ�����������Ҵ��⣬��������ɫ���塣�缫��Ӧ���£�
�������� 4OH����4e����O2��+2H2O
�� ��
�������� ��
��CH3CHO+2e��+2H2O��CH3CH2OH+2OH��
��3���������У�������Na2SO4�����ʵ��� ������������������С����������������
��4���������У�ijʱ�̲ⶨ����������Һ�и���ֵ����ʵ���������Na2SO4��CH3COOH�����ʵ�����ͬ�����й�����������Һ�и���Ũ�ȹ�ϵ��˵����ȷ���� ������ĸ��ţ���
a. c(Na+)��һ����c(SO42��)��2��
b. c(Na+)��2c(CH3COOH)+2c(CH3COO��)
c. c(Na+)+c(H+)��c(SO42��)+c(CH3COO��)+c(OH��)
d. c(Na+)��c(CH3COOH)��c(CH3COO��)��c(OH��)
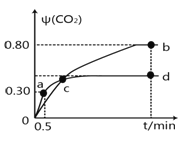
��5����֪����ȩ���Ҵ��ķе�ֱ�Ϊ20.8����78.4�����ӵ�������������Һ�з�����Ҵ���Ʒ�������� ��
��������1��CH4
��2��CH3CHO��2e����H2O��CH3COOH��2H��
2H����2e����H2����4H2O��4e����2 H2����4OH��
��3������
��4��abd
��5������
��������
�����������1������Դ��������������������b�缫���������������Լ���ȼ�ϵ��Ϊֱ����Դ����ȼ�ϵ����b��Ӧͨ��CH4���塣
��2������������ʧȥ���ӣ������������ŵ��⣬��ȩʧȥ����ת��Ϊ���ᣬ��Ӧ�ĵ缫��Ӧʽ��CH3CHO��2e����H2O��CH3COOH��2H���������õ����ӣ�������ȩ�õ�����ת��Ϊ�Ҵ����⣬��Һ�е�������Ҳ�õ�����ת��Ϊ�������缫��ӦʽΪ2H����2e����H2����
��3�������������������Ӳ�����缫��Ӧ������Ϊ���������ӽ���Ĥ����������Na2SO4�����ʵ���������
��4��a��������ͨ�������ӽ���Ĥ���������ƶ�����c(Na+)��һ����c(SO42��)��2����a��ȷ��b.���������غ��֪c(Na��)��2c(CH3COOH)��2c(CH3COO��)��b��ȷ��c�����ݵ���غ�c(Na��)��c(H��)��2c(SO42��)��c(CH3COO��)��c(OH��)��֪c(Na��)��c(H��)��c(SO42��)��c(CH3COO��)��c(OH��)�Ǵ���ģ�c����ȷ��d��������һԪ���ᣬ���볣��С������Һ��c(Na+)��c(CH3COOH)��c(CH3COO��)��c(OH��)��d
��ȷ����ѡabd.
��5����֪����ȩ���Ҵ��ķе�ֱ�Ϊ20.8 ����78.4 �������ڶ����ܣ������ӵ�������������Һ�з�����Ҵ���Ʒ�ķ���������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��Ϊ���о���������Թ�������ֽ����ʵ�Ӱ�죬ijͬѧ��������ʵ�飬��ش��������⡣
��� | ���� | ʵ������ |
�� | �ֱ����Թ�A��B�м���5mL 5% H2O2��Һ��������2�ε�Ũ�� FeCl3��Һ�����Թ��о����������ݳ���ʱ�����Թ�A����ʢ��5��������ˮ���ձ��н��ݣ����Թ�B����ʢ��40��������ˮ���ձ��н��� | �Թ�A�в��ٲ������ݣ��Թ�B�в��������������� |
�� | ��ȡ��֧�Թֱܷ����5mL 5% H2O2��Һ��5mL 10% H2O2��Һ | �Թ�A��B�о�δ���Լ��������ݲ��� |
��1����������ֽ�Ļ�ѧ����ʽΪ ��
��2��ʵ��ٵ�Ŀ���� ��
��3��ʵ���δ�۲쵽Ԥ�ڵ�ʵ������Ϊ�˰�����ͬѧ�ﵽʵ��Ŀ�ģ�������Ķ����������ĸĽ������ ����ʵ�����ṩ���Լ�����
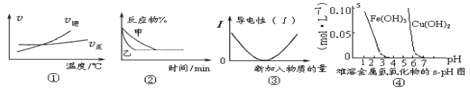
��4������H2O2�ֽⷴӦ��Cu2+Ҳ��һ���Ĵ����ã�Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ij��ѧ�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ������ش�������⣺
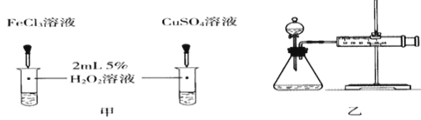
�ٶ��Է�������ͼ��ͨ���۲� �����ԱȽϵó���������ͬѧ�����FeCl3��ΪFe2(SO4)3��Ϊ�������������� ��
�ڶ�����������ͼ����ʾװ��������ʵ�飬ʵ��ʱ��������40 mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ʵ������Ҫ������������_________________________��
����Ŀ�����в��ֶ�����Ԫ�ص����ʻ�ԭ�ӽṹ���±���
Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
T | �������������ڲ����������4 |
X | �����������Ǵ�����������2�� |
Y | �����γɵ���̬�������ڱ�״���µ��ܶ�Ϊ0.76g��L-1 |
Z | Ԫ����������븺�۵ľ���ֵ֮����6 |
��1��Ԫ��X��һ��ͬλ�ؿɲⶨ�������������ͬλ�صķ����� �������ڱ��е�λ���� ��
��2��Ԫ��Y����Ԫ���γ�һ������YH4����д����������Z�����γɻ�����ĵ���ʽ ��
��3��д��Ԫ��X��T�γɵĻ�����XT2�ĵ���ʽ ��
��4��Ԫ��Z��Ԫ��T��ȣ��ǽ����Խ�ǿ���� ����Ԫ�ط��ű�ʾ�������б�������֤����һ��ʵ���� ������ţ���
a��������Z�ĵ��ʺ�T�ĵ���״̬��ͬ
b��Z���⻯���T���⻯���ȶ�
c��һ��������Z��T�ĵ��ʶ���������������Һ��Ӧ
d���������ȣ�Z������������T���ɶ�����
e��Tԭ����Zԭ�ӵ��Ӳ�����ͬ��Zԭ�Ӱ뾶С��Tԭ��
��5��T�ĵͼ�������ͨ��Z���ʵ�ˮ��Һ�У�������Ӧ�Ļ�ѧ����ʽΪ ��
��6���ֱ��õ���ʽ��ʾZ��þԪ�ء�Y�뵪Ԫ���γɻ�����Ĺ���: ��