��Ŀ����
����Ŀ��Ϊ���о���������Թ�������ֽ����ʵ�Ӱ�죬ijͬѧ��������ʵ�飬��ش��������⡣
��� | ���� | ʵ������ |
�� | �ֱ����Թ�A��B�м���5mL 5% H2O2��Һ��������2�ε�Ũ�� FeCl3��Һ�����Թ��о����������ݳ���ʱ�����Թ�A����ʢ��5��������ˮ���ձ��н��ݣ����Թ�B����ʢ��40��������ˮ���ձ��н��� | �Թ�A�в��ٲ������ݣ��Թ�B�в��������������� |
�� | ��ȡ��֧�Թֱܷ����5mL 5% H2O2��Һ��5mL 10% H2O2��Һ | �Թ�A��B�о�δ���Լ��������ݲ��� |
��1����������ֽ�Ļ�ѧ����ʽΪ ��
��2��ʵ��ٵ�Ŀ���� ��
��3��ʵ���δ�۲쵽Ԥ�ڵ�ʵ������Ϊ�˰�����ͬѧ�ﵽʵ��Ŀ�ģ�������Ķ����������ĸĽ������ ����ʵ�����ṩ���Լ�����
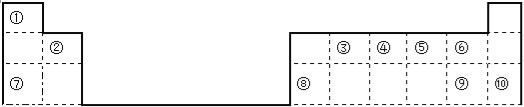
��4������H2O2�ֽⷴӦ��Cu2+Ҳ��һ���Ĵ����ã�Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ij��ѧ�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ������ش�������⣺

�ٶ��Է�������ͼ��ͨ���۲� �����ԱȽϵó���������ͬѧ�����FeCl3��ΪFe2(SO4)3��Ϊ�������������� ��
�ڶ�����������ͼ����ʾװ��������ʵ�飬ʵ��ʱ��������40 mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ʵ������Ҫ������������_________________________��
���𰸡���1��2H2O2 ![]() 2H2O+O2�� ��д��MnO2�����֣�
2H2O+O2�� ��д��MnO2�����֣�
��2���о��¶ȶ�H2O2�ֽ����ʵ�Ӱ��
��3������֧�Թ�ͬʱ����ʢ����ͬ�¶���ˮ���ձ��л� ����֧�Թ���ͬʱ����2��1mol/LFeCl3��Һ���۲�������ݵ�����
��4���� ��Һ�����ݲ��������ʣ��ų�Cl�� �ĸ��ţ��� �ų��������ӵ���ͬ�����ĸ��ţ��������ɣ� �� �ռ�40mL���������ʱ��
��������
�����������1����������ֽ�����ˮ���������÷�Ӧ�Ļ�ѧ����ʽΪ2H2O2 ![]() 2H2O+O2����
2H2O+O2����
��2������ʵ�����Ĺ��̿���֪������ʵ�����ڲ�ͬ���¶��½���ʵ�飬���Կ����жϸ�ʵ������֤�¶ȶԹ�������ֽ����ʵ�Ӱ�������ʵ��ٵ�Ŀ�����о��¶ȶ�H2O2�ֽ����ʵ�Ӱ����
��3������������Һ�ķֽ����ʽ��������Կ��Խ���������ͭ��Һ�������жϣ��ʿ������ʵ�����£�����֧�Թ�ͬʱ����ʢ����ͬ�¶���ˮ���ձ��У�������֧�Թ���ͬʱ������ͬ������Ũ��һ��������ͭ��Һ���������ݵ����ʿ����10%��H2O2��Һ����֮��5%��H2O2��Һ��
��4����������Է����������Ը��ݲ������ݵ������������жϷ�Ӧ�Ŀ��������ڼ�����Ȼ���������ͭ�������Ӳ�ͬ������Ϊ�ų������ӵĸ�����������FeCl3��ΪFe2(SO4)3��
���������������Ӧ�Ŀ�����������ͨ���ⶨ�ռ�40mL�����������ʱ�������ж���ʱ�����Ӧ����