��Ŀ����
2�����о�������Fe��SCN��3������Fe3+��SCN-��������1��3 �ĸ�������ϣ�������������������ϣ��밴Ҫ����գ���1��������Fe3+��SCN-��������У���Ҫ��Fe3+��SCN-�Ը�����1��1�������������Ѫ��ɫ�������ӵ����ӷ�����[Fe��SCN��]2+��
��2����Fe3+��SCN-�Ը�����1��5��ϣ���FeCl3��KSCN��ˮ��Һ�з�����Ӧ�Ļ�ѧ����ʽ���Ա�ʾΪFeCl3+5KSCN=K2[Fe��SCN��5]+3KCl��
���� ��1��Fe3+��SCN-�Ը�����1��1�����������Ϊ[Fe��SCN��]2+��
��2��Fe3+��SCN-�Ը�����1��5�����������Ϊ[Fe��SCN��5]2-����FeCl3��KSCN��ˮ��Һ�з�Ӧ����K2[Fe��SCN��5]��KCl
��� �⣺��1����Fe3+��SCN-�Ը�����1��1������ɴ�����������ɵ����ӣ�[Fe��SCN��]2+��
�ʴ�Ϊ��[Fe��SCN��]2+��
��2��Fe3+��SCN-�Ը�����1��5�����������Ϊ[Fe��SCN��5]2-����FeCl3��KSCN��ˮ��Һ�з�Ӧ����K2[Fe��SCN��5]��KCl�����Եķ�Ӧ����ʽΪ��FeCl3+5KSCN=K2[Fe��SCN��5]+3KCl��
�ʴ�Ϊ��FeCl3+5KSCN=K2[Fe��SCN��5]+3KCl��
���� ���⿼�����������γɼ�Ӧ�ã���Ŀ�ѶȲ�����ȷ��������ɡ��ṹ������Ϊ���ؼ���������ؿ���ѧ���ķ���������������֪ʶǨ��������

��ϰ��ϵ�д�
�����Ŀ
13��NA��ʾ�����ӵ�����������������ȷ���ǣ�������
| A�� | 0.5mol/L��Ba��0H��2��Һ��0Hһ����ĿΪNA | |
| B�� | 1 mol FeI2������������Ӧʱת�Ƶĵ�����Ϊ2NA | |
| C�� | 1.6gNH2-��������������ΪNA | |
| D�� | 92g NO2��N204��������к��е�ԭ����Ϊ6NA |
14���ܽ⡢���ˡ����������У����õ��������ǣ�������
| A�� | ��ֽ | B�� | �ձ� | C�� | �ƾ��� | D�� | ������ |
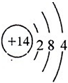 ��д��D2A2��C2A����ʽ��D2A2��
��д��D2A2��C2A����ʽ��D2A2��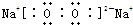 ��C2A��
��C2A�� ��
��