��Ŀ����
13��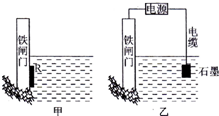 �������������������ʴ��ÿ����ʴ����ʧ�ĸֲ�ռ��������������$\frac{1}{4}$��
�������������������ʴ��ÿ����ʴ����ʧ�ĸֲ�ռ��������������$\frac{1}{4}$����1��Ϊ�˽���ijˮ�����բ�ű���ʴ�����ʣ����Բ�����ͼ��ʾ�ķ���������ԭ���ԭ�����ԭ��ء����ء���
��2��ͼ���ҷ���Ҳ�ɽ�����բ�Ÿ�ʴ���ʣ���Ϊ��ӵ�����������������������
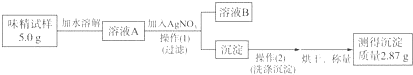
��3����5mol/L CuSO4��Һ200mL�ö��Ե缫���һ��ʱ������е�һ���缫�ռ�����״����448mL���壬Ϊʹ�������Һ�ָ�����ʼ��Ũ�ȣ�Ӧ��������Һ�м���3.2gCuO���ʣ��ѧʽ����
���� ��1����ԭ��ظ����Ľ������ٱ���ʴ����ԭ��������Ľ�����������
��2���ڵ��ص������ϵĽ��������������ݵ��صĹ���ԭ�����ش�
��3�����ݵ��صĹ���ԭ����Ҫ���õ���ĵ���ʸ�ԭ������ѭ��ԭ���ǣ���ʲô��ʲô�����������
��� �⣺��1��Ӧ��ԭ���ԭ������ԭ��ظ����Ľ������ٱ���ʴ����ԭ��������Ľ������������ʴ�Ϊ��ԭ��أ�
��2�����ص������ϵĽ�����������Ϊ������բ�ŵĸ�ʴ���ʣ�������բ��Ӧ��������ֱ����Դ�ĸ�������Ϊ��ӵ������������������ʴ�Ϊ����ӵ�����������������
��3���������������Ϊ448mL���壬���ʵ���Ϊ��$\frac{448mL��1{0}^{-3}L/ml}{22.4L/mol}$=0.02mol���ɵ�ⷴӦʽΪ��2H2O+2CuSO4$\frac{\underline{\;ͨ��\;}}{\;}$2Cu+O2��+2H2SO4 ��������������ͭ�����ʵ���Ϊ0.04mol��������ͭ�����ʵ�Ϊ��0.2L��5mol/L=1mol����������ͭδ��ȫ�ŵ磬����ֻҪ��������ͭ��ʹ�临ԭ������Ϊ��0.04mol��80g/mol=3.2g��d�ʴ�Ϊ��3.2��CuO��
���� ���⿼������ĸ�ʴ���������ȷԭ��غ͵���ԭ���ǽⱾ��ؼ������Բ���������ѧ����������������ֹ��������ʴ�������˷ѣ�

 ��У����ϵ�д�
��У����ϵ�д�| A�� | ����B��״ֻ̬��Ϊ��̬��Һ̬ | |
| B�� | ƽ��ʱ����λʱ����c��A�����ĩsc��C������=1�s1 | |
| C�� | ����������䣬��ƽ����ϵ�м���B��ƽ��������淴Ӧ�����ƶ� | |
| D�� | ����ʼʱ�������м���1molB��1molC���ﵽƽ��ʱ�ų�����a kJ |
| A�� | ����[KAl��SO4��2•12H2O]����ˮ���γɽ��壬��˿���������ˮ��ɱ������ | |
| B�� | úȼ��ʱ������������ʯ�ҿ��Լ��ٷ����ж��������ŷ� | |
| C�� | ������Ʒ��ͭ��Ʒ���ܷ���������ʴ���ܷ������ⸯʴ | |
| D�� | �۷���ϩ�Ǵ����� |
| A�� | ���ӻ�������һ���������Ӽ� | |
| B�� | ���ʷ����о������ڻ�ѧ�� | |
| C�� | ���м��Լ��ķ���һ�������Ǽ��Լ� | |
| D�� | ���й��ۼ��Ļ�����һ���ǹ��ۻ����� |

| A�� | ��������ỻ������֭�������в����е������� | |
| B�� | װ���д��ڡ���ѧ�ܡ����ܡ����ܡ���ת�� | |
| C�� | ͭƬ�������������� | |
| D�� | �����пƬ������Ƭ����·�еĵ������� |
| A�� | 1mol NH3���3NA��N-H�� | |
| B�� | 36g C60��������Ϊ0.05NA | |
| C�� | ��״���£�11.2L CO2�к���2NA�����õ��Ӷ� | |
| D�� | 18g��ˮ��${\;}_{1}^{2}$H2O���к���10NA������ |