��Ŀ����
�������Ļ�������������������������Ҫ����;��
(1) ��֪����26��Ԫ�أ�д��Fe�ļ۲���ӵ����Ų�ʽ________����֪��Ȼ������������ͬλ����������Ϊ30����ԭ�ӣ������ͬλ�ط���________��
(2) Feԭ�ӻ�������Χ�н϶���������Ŀչ�����������һЩ���ӻ������γ���������֮�γ������ķ��ӵ���λԭ��Ӧ�߱��Ľṹ������________��Fe(CO)3һ�������ɴ������һ�Ǧ��Ϊ���͵Ŀ����������������CO���ӡ�д��CO��һ�ֳ����ȵ�������ӵĽṹʽ________��������Ƚϣ��е���{����________�����ʽ����
(3) 1183K���´�������ľ�����ͼ1��ʾ��1183K������ת��Ϊͼ2��ʾ�����������־��������ڽ�����ԭ�Ӽ������ͬ��
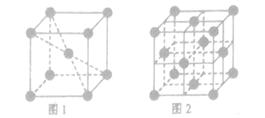
��ͼ1��ͼ2�У���ԭ�ӵ���λ��֮��Ϊ________��
�ڿռ���������ָ���ɾ����ԭ�ӡ����ӻ��������������ռ���ռ�е�����ٷֱȣ���ͼ1��ͼ2�У���ԭ�ӵĿռ�������֮��Ϊ________��
(1) ��֪����26��Ԫ�أ�д��Fe�ļ۲���ӵ����Ų�ʽ________����֪��Ȼ������������ͬλ����������Ϊ30����ԭ�ӣ������ͬλ�ط���________��
(2) Feԭ�ӻ�������Χ�н϶���������Ŀչ�����������һЩ���ӻ������γ���������֮�γ������ķ��ӵ���λԭ��Ӧ�߱��Ľṹ������________��Fe(CO)3һ�������ɴ������һ�Ǧ��Ϊ���͵Ŀ����������������CO���ӡ�д��CO��һ�ֳ����ȵ�������ӵĽṹʽ________��������Ƚϣ��е���{����________�����ʽ����
(3) 1183K���´�������ľ�����ͼ1��ʾ��1183K������ת��Ϊͼ2��ʾ�����������־��������ڽ�����ԭ�Ӽ������ͬ��

��ͼ1��ͼ2�У���ԭ�ӵ���λ��֮��Ϊ________��
�ڿռ���������ָ���ɾ����ԭ�ӡ����ӻ��������������ռ���ռ�е�����ٷֱȣ���ͼ1��ͼ2�У���ԭ�ӵĿռ�������֮��Ϊ________��
��1��3d64s2��2�֣��� ��2�֣� ��2�����йµ��Ӷԣ�2�֣���
��2�֣� ��2�����йµ��Ӷԣ�2�֣��� ��2�֣���CO��2�֣�
��2�֣���CO��2�֣�
��3��2�U3 ��2�֣� ��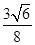 ����0.92�U1����3�֣�
����0.92�U1����3�֣�
 ��2�֣� ��2�����йµ��Ӷԣ�2�֣���
��2�֣� ��2�����йµ��Ӷԣ�2�֣��� ��2�֣���CO��2�֣�
��2�֣���CO��2�֣���3��2�U3 ��2�֣� ��
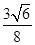 ����0.92�U1����3�֣�
����0.92�U1����3�֣������������1�����ݹ���ԭ����֪��Fe�ļ۲���ӵ����Ų�ʽΪ3d64s2���ڱ�ʾԭ�����ʱԪ�ط��ŵ����½DZ�ʾ�����������ϽDZ�ʾ�����������Ը����ķ�����
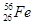 ��
����2���γ���λ����������������й¶Ե��ӣ�����ԭ�ӻ����Ӿ��пչ����������֮�γ������ķ��ӵ���λԭ��Ӧ�߱��Ľṹ�����Ǿ��йµ��Ӷԡ�ԭ�����ͼ۵������ֱ���ȵĶ��ǵȵ����壬������CO��һ�ֳ����ȵ���������ǵ�������ṹʽ��
 ��������CO�γɵľ��嶼�Ƿ��Ӿ��壬���е����ǷǼ��Է��ӣ�CO�Ǽ��Է��ӣ�����CO�ķ��Ӽ�������ǿ�ڵ����ģ���˷е�ϸߵ���CO��
��������CO�γɵľ��嶼�Ƿ��Ӿ��壬���е����ǷǼ��Է��ӣ�CO�Ǽ��Է��ӣ�����CO�ķ��Ӽ�������ǿ�ڵ����ģ���˷е�ϸߵ���CO����3���ٸ��ݾ����Ľṹ��֪��ͼ1����λ����8��ͼ2����λ����12��������λ��֮����2�U3��
������ԭ�Ӱ뾶��r��������߳�ͼ1��a��ͼ2��b�������ͼ1��֪a2��2a2��(4r)2�����a��
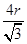 ������ͼ1����ԭ�ӵĿռ���������
������ͼ1����ԭ�ӵĿռ���������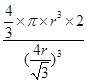 ������ͼ2��֪b2��b2��(4r)2�����b��
������ͼ2��֪b2��b2��(4r)2�����b��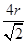 ������ͼ2����ԭ�ӵĿռ���������
������ͼ2����ԭ�ӵĿռ���������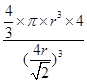 ��������ԭ�ӵĿռ�������֮��Ϊ
��������ԭ�ӵĿռ�������֮��Ϊ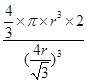 :
: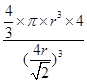 ��
��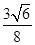 ��
��
��ϰ��ϵ�д�
�����Ŀ



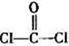 ���������______��
���������______��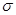 ����____��
����____��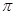 ��������Cԭ�ӵ��ӻ���ʽΪ_______��
��������Cԭ�ӵ��ӻ���ʽΪ_______��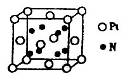 ��ʾ�������Ļ�ѧʽΪ_______��
��ʾ�������Ļ�ѧʽΪ_______��

