��Ŀ����
ﮡ�������ͭ��������Ļ���������Cu4O(PO4)2����ͨ�����з�Ӧ�Ʊ���
2Na3PO4+4CuSO4+2NH3��H2O=Cu4O(PO4)2��+3Na2SO4+(NH4)2SO4+H2O
��1��д����̬Cu2+�ĺ�������Ų�ʽ�� ��
��2��PO43���Ŀռ乹���� ��
��3����(N2H4)���ӿ���ΪNH3�����е�һ����ԭ�ӱ���NH2��������ȡ���γɵ���һ�ֵ����⻯�N2H4�����е�ԭ�ӹ�����ӻ������� ��
��4������CuSO4��5H2O�Ľṹʾ��ͼ���£��京�е������������� ��������ţ�

a�����Ӽ� b�����Լ� c��������
d����λ�� e����� f���Ǽ��Լ�
��5��������ͭ��Һ�м������KCN�����������[Cu(CN)4]2-����1 mol CN-�к��еĦм�����ĿΪ ��
��6��CuԪ����HԪ�ؿ��γ�һ�ֺ�ɫ������侧��ṹ��Ԫ��ͼ��ʾ����û�����Ļ�ѧʽΪ ��

2Na3PO4+4CuSO4+2NH3��H2O=Cu4O(PO4)2��+3Na2SO4+(NH4)2SO4+H2O
��1��д����̬Cu2+�ĺ�������Ų�ʽ�� ��
��2��PO43���Ŀռ乹���� ��
��3����(N2H4)���ӿ���ΪNH3�����е�һ����ԭ�ӱ���NH2��������ȡ���γɵ���һ�ֵ����⻯�N2H4�����е�ԭ�ӹ�����ӻ������� ��
��4������CuSO4��5H2O�Ľṹʾ��ͼ���£��京�е������������� ��������ţ�

a�����Ӽ� b�����Լ� c��������
d����λ�� e����� f���Ǽ��Լ�
��5��������ͭ��Һ�м������KCN�����������[Cu(CN)4]2-����1 mol CN-�к��еĦм�����ĿΪ ��
��6��CuԪ����HԪ�ؿ��γ�һ�ֺ�ɫ������侧��ṹ��Ԫ��ͼ��ʾ����û�����Ļ�ѧʽΪ ��

��1��[Ar]3d9 ��2���������� ��3��sp3 ��4��abde
��5��2NA��2mol ��6��CuH
��5��2NA��2mol ��6��CuH
��������� ��1��Cuԭ������Ϊ29����������Ų�ʽΪ[Ar]3d104s1��Cu2+�����Ų�ʽΪ[Ar]3d9����2��PO43����P�۲���Ӷ���=(5+0��4+3)/2=4��P��4��O�γ�4���������ӿռ乹��Ϊ�������塣��3��N2H4������N�γ�3��
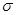 ������1�Թ¶Ե��ӣ�N�ӻ�����Ϊsp3����4��CuSO4��5H2O���Ϊ[Cu(H2O)4]SO4��H2O��[Cu(H2O)4]2+��SO42-�����Ӽ���ϣ�Cu2+��H2O����λ����ϣ�H��O�Լ��Թ��ۼ���ϣ��ᾧˮ�е�H��O�ֱ��O��H�γ��������5��CN-��C��N�γ���������������1��
������1�Թ¶Ե��ӣ�N�ӻ�����Ϊsp3����4��CuSO4��5H2O���Ϊ[Cu(H2O)4]SO4��H2O��[Cu(H2O)4]2+��SO42-�����Ӽ���ϣ�Cu2+��H2O����λ����ϣ�H��O�Լ��Թ��ۼ���ϣ��ᾧˮ�е�H��O�ֱ��O��H�γ��������5��CN-��C��N�γ���������������1��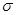 ����2��
����2��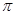 ������6��������Cu����Ϊ1/6��12+1/2��2+3=6��H����Ϊ1/3��6+4=6��
������6��������Cu����Ϊ1/6��12+1/2��2+3=6��H����Ϊ1/3��6+4=6�� ����
����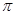 �� ��������
�� ��������
��ϰ��ϵ�д�
�����Ŀ

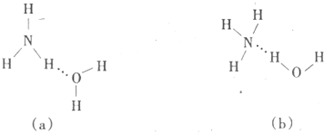



 )��
)��