��Ŀ����
��֪��I��L��M��Ϊ�����ĵ��ʣ�I�ڳ�����Ϊ���壬L��MΪ����������K��ˮ��Һ�Լ��ԣ�A����ǰ20��Ԫ����ɵ��������Ӹ�����Ϊ1��1�����ӻ�����������Ӻ���14�����ӣ�����һ�ֺ���ɫ�ķ�ĩ��GΪ��ɫ��״������D�ڳ���������ɫҺ�壬C����Һ��������ʵ���Ҽ���E��������֮���ת����ϵ����ͼ��ʾ(����������ͼ��û���г�)��

�����Ҫ����գ�
(1)A�Ļ�ѧʽΪ______________________��E�ĵ���ʽΪ_______________________��
(2)��Ӧ�ٵĻ�ѧ����ʽΪ___________________________________________________��
(3)��Ӧ�ڵĻ�ѧ����ʽΪ___________________________________________________��
(4)J��G�����ӷ���ʽΪ_____________________________________________________��

�����Ҫ����գ�
(1)A�Ļ�ѧʽΪ______________________��E�ĵ���ʽΪ_______________________��
(2)��Ӧ�ٵĻ�ѧ����ʽΪ___________________________________________________��
(3)��Ӧ�ڵĻ�ѧ����ʽΪ___________________________________________________��
(4)J��G�����ӷ���ʽΪ_____________________________________________________��
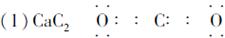
��2��H2O+C
 CO+H2
CO+H2��3��2Al+Fe2O3
 2Fe+Al2O3
2Fe+Al2O3��4��CO2+
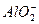 +2H2O====Al(OH)3��+
+2H2O====Al(OH)3��+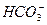 �����������𰸾��ɣ�
�����������𰸾��ɣ������ͻ�ƿ��ǡ�����һ�ֺ���ɫ�ķ�ĩ����֪����Fe2O3����L��MΪ�������ʿ�֪L��M�ֱ�ΪAl��Fe���Ƴ�I��J��D�ֱ�ΪH2��NaAlO2��H2O����GΪ��ɫ��״������֪GΪAl(OH)3��������K��ˮ��Һ�Լ��Կ�֪KΪNH3����ΪH��Fe2O3��Ӧ����Fe������H�л�ԭ�ԣ����Ƴ��ס�H��E�ֱ�ΪC��CO��CO2������B��һ������C��H����Ԫ�أ����ܺ���OԪ�أ���C����Һ��������ʵ���Ҽ���E(CO2)��֪CΪCa(OH)2��AΪCaC2��BΪC2H2��NΪ��Ρ�����ٴ�ͷ��β˳һ�顣

��ϰ��ϵ�д�
 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
�����Ŀ
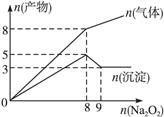
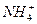 �Ļ��Һ�в��ȣ�������������������ʵ�����mol�������Na2O2�����ʵ�����mol���Ĺ�ϵ��ͼ��ʾ����ԭ��Һ��Al3+��Mg2+��
�Ļ��Һ�в��ȣ�������������������ʵ�����mol�������Na2O2�����ʵ�����mol���Ĺ�ϵ��ͼ��ʾ����ԭ��Һ��Al3+��Mg2+��