��Ŀ����
����Ŀ��������Ⱦ������������ȫ���ע��
��1��PM 2.5��ָ������ֱ��С�ڻ����2.5��m(1��m��100nm)�Ŀ��������˵������ȷ����_______(����ĸ����)��
a��PM 2.5��Ҫ��Դ�ڻ������硢��ҵ����������β���ŷŵȹ���
b��PM 2.5����С�����Զ�������
c��ֱ������1��2.5��m�Ŀ������ɢ�������п��γɽ���
d���ƹ�ʹ�õ綯���������Լ���PM2.5����Ⱦ
��2���������й�����������ʱ���о�������ʯ�������ҵȼúβ���е���(SO2��SO3)�͵�(NO��NO2)���¹��������¹��ռ��ܾ���β�������ܻ��Ӧ�ù㷺��CaSO4��Ca(NO2)2��
��CaSO4���Ե���ˮ���Ӳ��ʱ�䣬β����SO2��ʯ���鷴Ӧ����CaSO4�Ļ�ѧ����ʽΪ_______________________________________________________��
��Ca(NO2)2���Ƴɻ��������������ֽ�������ȡ�β����NO��NO2��ʯ���鷴Ӧ����Ca(NO2)2�Ļ�ѧ����ʽΪ__________________________________________��
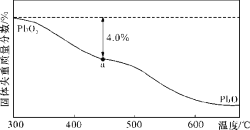
��3�������е�Ǧ��Ⱦ���û���ԭ�����շֹ��ȷ���������֪PbO2�ڼ��ȹ����з����ֽ��ʧ����������ͼ��ʾ����֪ʧ�������ϵ�a��Ϊ��Ʒʧ��4.0% ![]() �IJ�����������a������ʾΪPbOx��mPbO2��nPbO����ʽ����xֵ��m��nֵ��(�������һλС��)(Ҫ��д���������)��____________
�IJ�����������a������ʾΪPbOx��mPbO2��nPbO����ʽ����xֵ��m��nֵ��(�������һλС��)(Ҫ��д���������)��____________
 ��
��
���𰸡�bc 2SO2��O2��2Ca(OH)2===2CaSO4��2H2O NO��NO2��Ca(OH)2===Ca(NO2)2��H2O PbO2====PbOx + ![]() O2 ��
O2 �� ![]() ��32 == 239 �� 4.0% x==1.4
��32 == 239 �� 4.0% x==1.4 ![]() == 1.4
== 1.4 ![]() ==
== ![]()
��������
��1��a. PM2.5��ָ������ֱ��С�ڻ����2.5��m�Ŀ����������Ҫ��Դ���ճ����硢��ҵ����������β���ŷŵȹ����о���ȼ�ն��ŷŵIJ����
b. PM2.5��������������ԣ������������ж����ʡ�
c.������ֱ����10-7m~ 10 -9m֮�䣬 PM2.5���ӵĴ�С�����ϡ�
d.���ٻ�����β���ŷţ��������̳����ܽ��Ϳ�����PM2.5��
��2����β����SO2��������ʯ���鷴Ӧ����CaSO4��ˮ��
��β����NO��NO2 ��ʯ���鷴Ӧ����Ca(NO2)2��ˮ��
��3����a�������ɱ�ʾΪPbOx������PbO2=PbOx + ![]() O2�����У�1-2/x����32=239��4.0%����x=1.4�������ΪmPbO2��nPbO������ԭ���غ�ã�Oԭ�Ӻ�Pbԭ�ӵı�ֵ=
O2�����У�1-2/x����32=239��4.0%����x=1.4�������ΪmPbO2��nPbO������ԭ���غ�ã�Oԭ�Ӻ�Pbԭ�ӵı�ֵ=![]() = 1.4��
= 1.4��![]() =
= ![]() ����ͨ���������x=1.4��
����ͨ���������x=1.4��![]() =
= ![]() ��
��
��1��a. PM2.5��ָ������ֱ��С�ڻ����2.5��m�Ŀ����������Ҫ��Դ���ճ����硢��ҵ����������β���ŷŵȹ����о���ȼ�ն��ŷŵIJ������a��ȷ��
b. PM2.5��������������ԣ������������ж����ʣ���b����
c.������ֱ����10-7m~ 10 -9m֮�䣬 PM2.5���ӵĴ�С�����ϣ���c����
d.���ٻ�����β���ŷţ��������̳����ܽ��Ϳ�����PM2.5����d��ȷ��
��ѡ��bc��
��2����β����SO2��������ʯ���鷴Ӧ����CaSO4��ˮ,��Ӧ����ʽΪ��2SO2��O2��2Ca(OH)2=2CaSO4��2H2O���ʴ�Ϊ��2SO2��O2��2Ca(OH)2=2CaSO4��2H2O��
��β����NO��NO2 ��ʯ���鷴Ӧ����Ca(NO2)2��ˮ����Ӧ����ʽΪNO��NO2��Ca(OH)2=Ca(NO2)2��H2O���ʴ�Ϊ��NO��NO2��Ca(OH)2=Ca(NO2)2��H2O��
��3����a�������ɱ�ʾΪPbOx������PbO2=PbOx + ![]() O2�����У�1-2/x����32=239��4.0%����x=1.4�������ΪmPbO2��nPbO������ԭ���غ�ã�Oԭ�Ӻ�Pbԭ�ӵı�ֵ=
O2�����У�1-2/x����32=239��4.0%����x=1.4�������ΪmPbO2��nPbO������ԭ���غ�ã�Oԭ�Ӻ�Pbԭ�ӵı�ֵ=![]() = 1.4��
= 1.4��
![]() =
= ![]() ����ͨ���������x=1.4��
����ͨ���������x=1.4��![]() =
= ![]() ���ʴ�Ϊ��PbO2=PbOx +
���ʴ�Ϊ��PbO2=PbOx + ![]() O2
O2 ![]() ��32 == 239 �� 4.0% x=1.4
��32 == 239 �� 4.0% x=1.4 ![]() = 1.4
= 1.4 ![]() =
= ![]() ��
��

 �����ߴ���ϵ�д�
�����ߴ���ϵ�д�����Ŀ��Ϊ��֤������Cl2��Fe3+��SO2��ijС������ͼ��ʾװ�ý���ʵ�飨�г������ͼ��м���װ�����ԣ��������Ѽ��飩��

ʵ�鲽�裺
���ڼ�װ���У�����a�����ȣ���װ���г�������ɫ����ʱ�����װ�����ӡ�
�ڵ���װ����FeC12��Һ���ʱ��ֹͣ���ȡ�
�۴���c��ʹԼ2mL����Һ�����Թ��У�������Һ�е����ӡ�
������װ���У�����b���������ž������в���������ͨ��������װ�ñ�ƺ����Һ�У�һ��ʱ���ֹͣ��
�ݸ��±����Թܣ�����c��ʹԼ2mL����Һ�����Թ��У�������Һ�е����ӡ�
�ش��������⣺
��1�����з�����Ӧ�Ļ�ѧ����ʽΪ______________________��
��2����70%��������ȡSO2����Ӧ���ʱ���98%������죬ԭ����_________________��
��3��ʵ���У�֤��������Fe3+��SO2�����ӷ���ʽΪ______________________________��
��4���Т�����ͬѧ�ֱ����������ʵ�飬ʵ�������£�
����3��Һ�к��е����� | ����5��Һ�к��е����� | |
�� | ����Fe3+����Fe2+ | ��SO42- |
�� | ��Fe3+��Fe2+ | ��SO42- |
�� | ��Fe3+��Fe2+ | ��Fe2+ |
����ʵ����һ���ܹ�֤��Cl2��Fe3+��SO2����______���I������II����III������