��Ŀ����
����Ŀ����1����֪C(s�����ʯ)+O2(g)![]() CO2(g)����H=��395.4 kJ��mol��1��C(s��ʯī)��O2(g)
CO2(g)����H=��395.4 kJ��mol��1��C(s��ʯī)��O2(g)![]() CO2(g)����H=��393.5 kJ��mol��1��
CO2(g)����H=��393.5 kJ��mol��1��
��ʯī�ͽ��ʯ��ȣ�ʯī���ȶ���_________���ʯ���ȶ��ԡ�
��ʯī��C��C������________���ʯ��C��C�����ܡ�(������ڡ� ��С�ڡ����ڡ�)��
��2����4g CH4��ȫȼ��������̬CO2��Һ̬ˮ���ų�����222.5 kJ�����Ȼ�ѧ��Ӧ����ʽΪ��______________________________________________________��
��3��0.5mol����̬����ȼ��������(B2H6)��������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�649.5kJ���������Ȼ�ѧ��Ӧ����ʽΪ��______________________________________________��
��4����֪���з�Ӧ�ķ�Ӧ�ȣ�
CH4(g)��H2O(g)![]() CO(g)��3H2(g) ��H1��+206.2kJ��mol��1
CO(g)��3H2(g) ��H1��+206.2kJ��mol��1
CH4(g)��CO2(g)![]() 2CO(g)��2H2(g) ��H2����247.4 kJ��mol��1
2CO(g)��2H2(g) ��H2����247.4 kJ��mol��1
��CH4(g)��H2O(g)��Ӧ����CO2(g)��H2(g)���Ȼ�ѧ����ʽ______________________________��
���𰸡����ڴ��� CH4(g)+2O2(g)![]() CO2(g)+2 H2O(l) ��H����890 kJ��mol��1 B2H6(g) +3O2(g)
CO2(g)+2 H2O(l) ��H����890 kJ��mol��1 B2H6(g) +3O2(g)![]() B2O3(g)+3H2O(l) ��H����1299 kJ��mol��1CH4(g)��2H2O(g)
B2O3(g)+3H2O(l) ��H����1299 kJ��mol��1CH4(g)��2H2O(g)![]() CO2(g)��4H2(g) ��H��+659.8 kJ��mol��1
CO2(g)��4H2(g) ��H��+659.8 kJ��mol��1
��������
��1��C��s�����ʯ��+O2��g���TCO2��g����H=-395.4 kJ��mol��1����C��s��ʯī��+O2��g���TCO2��g����H=-393.5 kJ��mol��1���ɸ�˹���ɿ�֪����-�ڵõ�C��s�����ʯ���TC��s��ʯī����
��2��n��CH4��=4g/16g��mol��1=0.25mol����֪1molCH4��ȫȼ��������̬CO2��Һ̬ˮ���ų�����Ϊ222.5kJ/0.25=890kJ��������ʵ�״̬���ʱ���д�Ȼ�ѧ����ʽ��
��3��0.5mol����̬����ȼ��������(B2H6)��������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�649.5kJ��������1mol��̬����ȼ����������������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�1299KJ��������
��4�����ݸ�˹���������㷴Ӧ���ʱ䡣
��1����C��s�����ʯ��+O2��g���TCO2��g����H=-395.4 kJ��mol��1����C��s��ʯī��+O2��g���TCO2��g����H=-393.5 kJ��mol��1���ɸ�˹���ɿ�֪����-�ڵõ�C��s�����ʯ���TC��s��ʯī������H=-395.4 kJ��mol��1-��-393.5 kJ��mol��1��=-1.9 kJ��mol��1�����ʯ�����ߣ�ʯī�ȶ��Դ��ڽ��ʯ����ʯī��C��C�����ܴ��ڽ��ʯ��C��C�����ܡ�
(2)n��CH4��=4g/16g��mol��1=0.25mol����֪1molCH4��ȫȼ��������̬CO2��Һ̬ˮ���ų�����Ϊ222.5kJ/0.25=890kJ�������ʵ�״̬���ʱ�Ϊ����֪�Ȼ�ѧ����ʽCH4��g��+2O2��g���TCO2��g��+2 H2O��l����H=-890 kJ��mol��1.
��3��0.5mol����̬����ȼ��������(B2H6)��������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�649.5kJ��������1mol��̬����ȼ����������������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�1299KJ����������Ӧ���Ȼ�ѧ����ʽΪ��B2H6(g) +3O2(g)![]() B2O3(g)+3H2O(l) ��H����1299 kJ��mol��1��
B2O3(g)+3H2O(l) ��H����1299 kJ��mol��1��
��4����CH4(g)��H2O(g)![]() CO(g)��3H2(g) ��H1��+206.2kJ��mol��1
CO(g)��3H2(g) ��H1��+206.2kJ��mol��1
��CH4(g)��CO2(g)![]() 2CO(g)��2H2(g) ��H2����247.4 kJ��mol��1
2CO(g)��2H2(g) ��H2����247.4 kJ��mol��1
���ݸ�˹���������㷴Ӧ���ʱ����2������CH4(g)��2H2O(g)![]() CO2(g)��4H2(g) ��H��+659.8 kJ��mol��1��
CO2(g)��4H2(g) ��H��+659.8 kJ��mol��1��

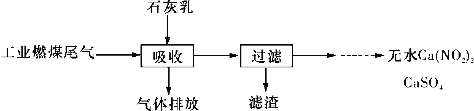
����Ŀ���Ȱ�����������������Ӧ���ɵ�һ�����dz��õ�����ˮ��������������Ҫ����һ�Ȱ������Ȱ������Ȱ�(NH2C1��NHC12��NC13)����������������ˮ��������
�ش��������⣺
(1)��һ�Ȱ�(NH2Cl)�ĵ���ʽΪ__________��
�ڹ�ҵ�Ͽ����÷�ӦCl2(g)+NH3(g)=NH2Cl(l)+HCl(g)�Ʊ�һ�Ȱ�����֪���ֻ�ѧ���ļ������±���ʾ�����費ͬ������ͬ�ֻ�ѧ����������ͬ������÷�Ӧ�ġ�H=______________��
��ѧ�� | N-H | Cl-Cl | N-Cl | H-Cl |
����(kJ/mol) | 391.3 | 243.0 | 191.2 | 431.8 |
��һ�Ȱ�����Ҫ��ˮ����������ԭ��������һ�Ȱ������ԡ����Ի����лᷢ��ˮ�⣬���ɾ���ǿ��ɱ�����õ����ʣ��÷�Ӧ�Ļ�ѧ����ʽΪ_________________��
(2)��Cl2��NH3��Ӧ�Ʊ����Ȱ��ķ���ʽΪ2Cl2(g)+NH3(g)![]() NHCl2(g)+2HCl(g)�����ݻ���Ϊ1 L�ļס����������£���Ӧ�¶ȷֱ�Ϊ400�桢T��)�����зֱ����2 mol C12��2 mol NH3����ø�������n(Cl2)�淴Ӧʱ��t�ı仯������±���ʾ��
NHCl2(g)+2HCl(g)�����ݻ���Ϊ1 L�ļס����������£���Ӧ�¶ȷֱ�Ϊ400�桢T��)�����зֱ����2 mol C12��2 mol NH3����ø�������n(Cl2)�淴Ӧʱ��t�ı仯������±���ʾ��
t/min | 0 | 40 | 80 | 120 | 160 |
n(Cl2)(������)/mol | 2.00 | 1.50 | 1. 10 | 0.80 | 0.80 |
n(Cl2) (��������/mol | 2.00 | 1.45 | 1.00 | 1.00 | 1.00 |
�ټ������У�0��40 min����NH3��Ũ�ȱ仯��ʾ��ƽ����Ӧ����v(NH3)=______________��
�ڸ÷�Ӧ�ġ�H________0(�>����<��) ��������____________________��
�۶Ը÷�Ӧ������˵����ȷ����______________(��ѡ����ĸ����
A.�������������ܶȲ��䣬�������Ӧ�ﵽƽ��״̬
B.��������C12��NH3���ʵ���֮�Ȳ��䣬�������Ӧ�ﵽƽ��״̬
C.��Ӧ�ﵽƽ��������������䣬��ԭ�����г���һ����������Cl2��ת��������
D.��Ӧ�ﵽƽ��������������䣬����һ������NHCl2��ƽ�����淴Ӧ�����ƶ�
(3)�ں��������£�2molCl2��1molNH3������Ӧ2Cl2(g)+NH3(g)![]() NHCl2(l)+2HCl(g)�����ƽ��ʱCl
NHCl2(l)+2HCl(g)�����ƽ��ʱCl

��A��B��C������Cl2ת������ߵ���______��(�A����B����C��)��
�ڼ���C��ʱ�÷�Ӧ��ѹǿƽ�ⳣ��Kp(C)=_______(Kp��ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ=��ѹ�����ʵ�������)