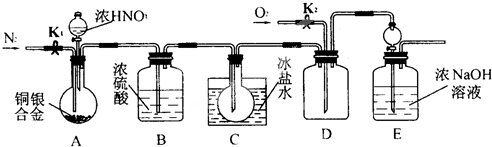
��Ŀ����
18�������Ƕ�ijˮ��Һ�������Ӽ���ķ����ͽ��ۣ�������ȷ���ǣ�������| A�� | �ȼ���BaCl2��Һ���ټ���������HNO3��Һ�������˰�ɫ��������Һ��һ�����д�����SO42- | |
| B�� | ����������CaCl2��Һ�������˰�ɫ��������Һ��һ���д�����CO32- | |
| C�� | �ýྻ�IJ�˿պȡ����Һ�����ڻ��������գ�����ɫ�ܲ����ܹ۲쵽�������ɫ������Һ��һ�����м����ӣ����ܺ��������� | |
| D�� | �ȼ����������Ὣ��Һ�ữ���ټ�AgNO3��Һ�������˰�ɫ��������Һ��һ�����д�����Cl- |
���� A���ð�ɫ��������Ϊ�Ȼ�����ԭ��Һ�п��ܴ��������ӣ���һ��������������ӣ�
B������CaCl2��Һ������ɫ���������ӣ������������ӣ�
C������ɫ�ܲ����ܹ۲쵽�������ɫ������Һ��һ�����м����ӣ����ܴ��������ӣ�
D������Һ���������ữ����ʱ�������������ӣ�����֤��ԭ��Һ�д��������ӣ�
��� �⣺A����ij��Һ�м����Ȼ�����Һ���а�ɫ�������ɣ��ټ������ữ���������ܽ⣬�ð�ɫ��������Ϊ�Ȼ�����ԭ��Һ�п��ܺ��������ӣ���һ��������������ӣ���A����
B������CaCl2��Һ������ɫ���������ӣ������������ӣ���˵������Һ��һ���д�����CO32-����B����
C������ɫ�ܲ����ܹ۲쵽�������ɫ������Һ��һ�����м����ӣ����ܴ��������ӣ���C��ȷ��
D������Һ���������ữ����ʱ�������������ӣ�����֤��ԭ��Һ�д��������ӣ���D����ѡC��
���� ������Ҫ������dz��������Ӻ������ӵļ��飬����һ�������Ƿ���ڣ�Ҫ�ų��������Ƶ����ӵĸ��ţ������ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
8����amol������Ͷ�뺬HNO3bmol��ϡ��Һ�ǡ����ȫ��Ӧ���ų�NO���壬��a��b�Ĺ�ϵ�ǣ�������
| A�� | $\frac{a}{b}$=$\frac{1}{4}$ | B�� | $\frac{a}{b}$=$\frac{3}{8}$ | C�� | $\frac{1}{4}$��$\frac{a}{b}$��$\frac{3}{8}$ | D�� | $\frac{a}{b}$��ֵ��ȷ�� |
13�����ڻ�ѧ��Ӧ��Ӧ������˵�������û���Ӧ����������ԭ��Ӧ���ڸ��ֽⷴӦ������������ԭ��Ӧ�����û���Ӧ������������ԭ��Ӧ���ֽܷⷴӦ������������ԭ��Ӧ�����е��ʲμӵķ�Ӧһ����������ԭ��Ӧ��������ȷ���ǣ�������
| A�� | �٢ڢۢ� | B�� | �٢ڢ� | C�� | �٢ڢ� | D�� | �ۢܢ� |
7��Ϊ�������ڿ�����Ⱦ�����д�ʩ��ȷ���ǣ�������
| A�� | ��������ֲ«���������ٵ���ɫֲ�� | |
| B�� | ���������װ�� | |
| C�� | ��Ҫ�����Ŵ���ע�⾭������ͨ�� | |
| D�� | ��������ζʱ��Ҫ��ʱ��������ˮ |
8�����ó���������ˮ��ϴ���ú����ֺ���ɫ����ߣ��ڴ˹����в������Ļ�ѧ��Ӧ�ǣ�������
| A�� | Fe-3e-=Fe3+ | B�� | 2Fe+2H2O+O2=2Fe��OH��2�� | ||
| C�� | 2H2O+O2+4e-=4OH- | D�� | 4Fe��OH��2+2H2O+O2=4Fe��OH��3 |
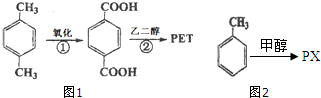 �Զ��ױ���Ӣ������p-xylene����дΪPX����һ�ֵͶ������Ҳ�Ǿ�����ҵ����Ҫԭ�ϣ���Ҫ���������Ա������ᣨPTA�����Ա��������ٺ��Ҷ�����EG����Ӧ���ɾ۶Ա��������Ҷ�������PET�������ڶԶ��ױ��������������Ż�����Ӧȱ�ڼӴ�һ���PX�������ڣ���PXΪ��Ҫԭ������PET��һ��·����ͼ1��
�Զ��ױ���Ӣ������p-xylene����дΪPX����һ�ֵͶ������Ҳ�Ǿ�����ҵ����Ҫԭ�ϣ���Ҫ���������Ա������ᣨPTA�����Ա��������ٺ��Ҷ�����EG����Ӧ���ɾ۶Ա��������Ҷ�������PET�������ڶԶ��ױ��������������Ż�����Ӧȱ�ڼӴ�һ���PX�������ڣ���PXΪ��Ҫԭ������PET��һ��·����ͼ1��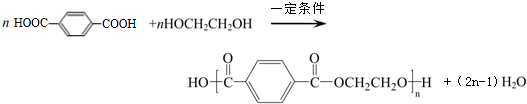 ��������ע��������
��������ע��������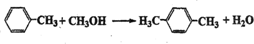 ��������ע��������
��������ע��������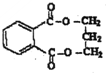 ��
��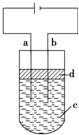 ����Fe��OH��2���ױ�����������ʵ���Һ�������������Һ���ռӦ�Ƶð�ɫ��������Fe��OH��2������Ӧ����ͼ��ʾ���ʵ����Ƶð�ɫ��������Fe��OH��2���������缫�IJ��Ϸֱ�Ϊʯī������
����Fe��OH��2���ױ�����������ʵ���Һ�������������Һ���ռӦ�Ƶð�ɫ��������Fe��OH��2������Ӧ����ͼ��ʾ���ʵ����Ƶð�ɫ��������Fe��OH��2���������缫�IJ��Ϸֱ�Ϊʯī������