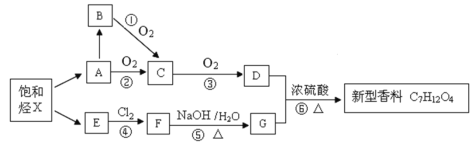
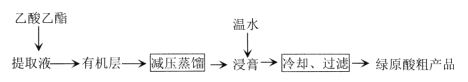
��Ŀ����
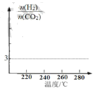
����Ŀ��ijͬѧȡһ�������������Ͻ���100 mLxmol/Lϡ�����ַ�Ӧ����Ӧ������û������ų����ڷ�Ӧ���������Һ�У���μ���2 mol/L NaOH��Һ������ NaOH��Һ�����(mL)��������������ʵ���(mol)�Ĺ�ϵ��ͼ��ʾ��
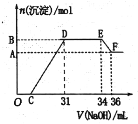
(1)д��EF�η�����Ӧ�����ӷ���ʽ____________
(2)C���Ӧ����Һ�к��е������ӳ�Na+�⣬������_______��
(3)x =________��
(4)�����Ͻ����������ʵ�������Ϊ_________��
���𰸡�Al��OH��3+OH-=AlO2-+2H2O NH4+��Al3+��Fe3+ 0.74 25%
��������
���ۺ����۵Ļ������100mLxmol/LϡHNO3��ַ�Ӧ��������ΪAl3+��Fe3+��ͨ�����⣬��Ӧ������û������ų���˵��û�е������������ɣ����ͼ��NԪ����+5�����-3�ۣ�������笠����ӡ���ͼ�ɵ������������������������ҺӦ�������ᷴӦ�������ɳ�������������ȫ����ͼ֪������������������Һ�����������䣬����������NH4+�����˷�Ӧ��������NaOH�ĵμӣ������ķ�Ӧ�����У�H++OH-=H2O��Fe3++3OH-=Fe(OH)3����Al3++3OH-=Al(OH)3����NH4++OH-�TNH3H2O��Al(OH)3+OH-=AlO2-+2H2O���ݴ˷������
(1)E��F֮������������٣�����ΪAl(OH)3�ܹ�������������Һ���ܽ⣬��Ӧ�����ӷ���ʽΪAl(OH)3+OH-=AlO2-+2H2O���ʴ�Ϊ��Al(OH)3+OH-=AlO2-+2H2O��
(2)���ۺ����۵Ļ������һ����ϡHNO3��ַ�Ӧ��������ΪAl3+��Fe3+��ͨ�����⣬��Ӧʼ��û���������ɣ���ͼ�ɵ������������������������ҺӦ�������ᷴӦ������C�㺬��Na+����������ȫ����ͼ֪������������������Һ�����������䣬Ϊ����������NH4+�����˷�Ӧ��NH4++OH-�TNH3H2O��������Һ�д���NH4+����C���Ӧ����Һ�к��е������ӳ�Na+�⣬������NH4+��Al3+��Fe3+���ʴ�Ϊ��NH4+��Al3+��Fe3+��
(3)E��F֮������������٣�ΪAl(OH)3�ܽ�������������Һ�У�
�䷴Ӧ�ķ���ʽΪAl(OH)3+NaOH=NaAlO2+2H2O�����������������������ʵ���֮��Ϊ1��1��EF�ε�n(NaOH)=2mol/L��(36-34)��10-3L=0.004mol������Al��Al(OH)3��NaOH��NaAlO2���������ʵ���Ϊ0.004mol�����������ᷴӦû�����������˵����ԭ����Ϊ����泥�DE��ΪNH4NO3��NaOH��Ӧ������NH4NO3��NaOH��n(NH4NO3)=n(NaOH)=(34-31)��10-3L��2mol/L=0.006mol������E��ʱ����Һ�е������������ƺͰ�ˮ��n(NH3H2O )=n(NH4+)=0.006mol��n(NaNO3)=n(NaOH)=34��10-3L��2mol/L=0.068mol�����ݵ�ԭ���غ�֪��c(HNO3)=![]() =
=![]() =0.74mol/L���ʴ�Ϊ��0.74��
=0.74mol/L���ʴ�Ϊ��0.74��
(4)��������ʼ�����ᷴӦ��ʱ������+3�����ӣ�NԪ�ر��笠����ӡ�������ymol��
Al����Ϊ0.004mol��NH4+Ϊ0.006mol�����ݵ�ʧ�����غ㣺3y+0.004��3=0.006��8����ã�y=0.012mol������������Ϊ0.012mol������ԭ���غ�֪���������ʵ�����0.012mol�������Ͻ����������ʵ�������Ϊ![]() ��100%=25%���ʴ�Ϊ��25%��
��100%=25%���ʴ�Ϊ��25%��