��Ŀ����
�������п�ͼ�ش�����(����ʱ,����ʽ�е�M��E������Ӧ��Ԫ�ط��ű�ʾ):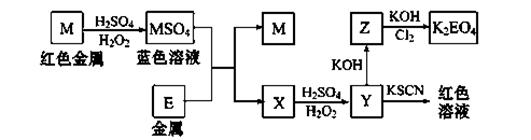
��1��д��M����ϡ�����H2O2���Һ�Ļ�ѧ����ʽ:�� ��
��2��д��X��Y�����ӷ���ʽ:�� ��
��3��д��Cl2��Z����ΪK2EO4�Ļ�ѧ����ʽ:�� ��
��K2EO4��һ�����͵�����ˮ������,�������ʺ������� ��
| A����ǿ������,������ɱ��,��ԭ����������ˮ������ |
| B����ǿ��ԭ��,������ɱ��,��������������ˮ������ |
| C����ǿ������,������ˮ������,��ԭ����������ɱ�� |
| D����ǿ��ԭ��,������ˮ������,��������������ɱ�� |
��1��Cu + H2O2 + H2SO4=CuSO4 + 2H2O ��2��2Fe2+ + H2O2+2H+ =2Fe3+ +2H2O ��3�� ��10KOH + 3Cl2+2Fe(OH)3=2K2FeO4+6KCl+8 H2O��A
���������������1������MΪ��ɫ������MSO4Ϊ��ɫ��Һ�������Ƴ�MΪCu������д����Ӧ�ķ���ʽCu + H2O2 + H2SO4=CuSO4 + 2H2O��2��X��H2O2�����±��Y�������Ƴ�Fe2+������ΪFe3+��3��ZΪFe(OH)3��Cl2��������ΪK2FeO4���������������ʣ������ǿ�����ԣ�����Fe(OH)3������������ԡ�����ѡ��Aѡ�
���㣺�����������ʽ����ƶϣ��������غ���ƽ����ʽ��

��ϰ��ϵ�д�
�����Ŀ
����ʵ������ܹ��ﵽĿ�ĵ���
| ѡ�� | ʵ��Ŀ�� | ʵ����� |
| A | ֤��Ksp(AgCl)��Ksp(AgI) | ��AgCl����Һ�е���KIŨ��Һ |
| B | ��ȥCu���е�CuO | �������еμ�����ϡ���� |
| C | ������Һ���Ƿ���Fe2�� | ����Һ�е���KSCN��Һ���ٵμ���ˮ |
| D | ֤��H2CO3���Ա�H2SiO3ǿ | Na2CO3��SiO2�ڸ��������ڷ�Ӧ |


 ��+����δ��ƽ������ƽ����ټ�����B����ƽ�� �ƶ�������� ����������������ƽ�����ԭƽ����ȣ�B��ת���� ������� ����С�����䡱����
��+����δ��ƽ������ƽ����ټ�����B����ƽ�� �ƶ�������� ����������������ƽ�����ԭƽ����ȣ�B��ת���� ������� ����С�����䡱����