��Ŀ����
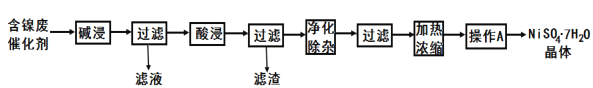
����Ŀ��ij����(Ni)�ϴ�������Ҫ����Ni��������Al��Al2O3��Fe�������������ᡢ������ʡ����ֽ�����������Ksp����ֵ���±���ʾ��
��ѧʽ | Fe(OH)2 | Fe(OH)3 | Al(OH)3 | Ni(OH)2 |
Ksp����ֵ | 10-17 | 10-39 | 10-34 | 10-15 |
���ú����ϴ����Ʊ�NiSO4��7H2O���壬������ͼ��ͼ��
�ش��������⣺
��1����Һ�е�������Ϊ_____��
��2�����������ʹ�õ���Ϊ_____��
��3����������������Ϊ�˳����������H2O2��Һ����Һ����ػ�ɫ��������Ӧ�����ӷ���ʽΪ__��һ��ʱ�����Һ�������ݳ��֣������ȣ�����к��ɫ�������ɡ��������ݵ�ԭ����__��Ȼ�����pH�Ϳ�ʹ��Һ����Ԫ����ȫ��������ʹ��պó�����ȫ������Ũ��Ϊ1.0��10-5mol/L������ʱ��pH=__������һλС����
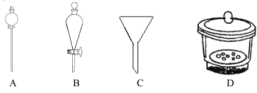
��4��������A��Ϊ_____�����ˡ�ϴ�ӡ�������ò�Ʒ��
��5���������������������˵����ȷ����__(�����)��
������FeCl3��AlCl3��Һʱ�������Ƚ�����FeCl3��AlCl3���ڽ�Ũ�����ᣬ��������ˮϡ�͵�����Ũ��
��FeCl2��FeCl3��Fe(OH)3������ͨ�����Ϸ�Ӧ����
��������ˮ��KSCN��Һ���Լ���FeCl3��Һ������Fe2+
�ܼ�������Al2(SO4)3��Һ��������ijɷ�ΪAl2O3
���𰸡�AlO2-��OH- ϡ���ᣨ��H2SO4�� H2O2+2Fe2++2H+=2H2O+2Fe3+ Fe3+��H2O2�ֽ����O2 2.7 ��ȴ�ᾧ �٢�
��������
ij����![]() �ϴ�������Ҫ����Ni��������Al��
�ϴ�������Ҫ����Ni��������Al��![]() ��Fe�������������ᡢ������ʣ��������̵�Ŀ�����ú����ϴ����Ʊ�
��Fe�������������ᡢ������ʣ��������̵�Ŀ�����ú����ϴ����Ʊ�![]() ���壬�����������̣�����Ni�ϴ��������Al��
���壬�����������̣�����Ni�ϴ��������Al��![]() ���ڼ�����
���ڼ�����![]() �����й��˲�������Fe��Ni�����������ڼ�����ʱ����˳��������������Fe��Ni�������γ�
�����й��˲�������Fe��Ni�����������ڼ�����ʱ����˳��������������Fe��Ni�������γ�![]() ��
��![]() �����й��˲�����������������ʱ����˳�����Һ����Ҫ����
�����й��˲�����������������ʱ����˳�����Һ����Ҫ����![]() ��
��![]() ������������Ҫ��ȥFeԪ�صõ�NiԪ�ص���Һ���������������õ�
������������Ҫ��ȥFeԪ�صõ�NiԪ�ص���Һ���������������õ�![]() ���塣
���塣
![]() �������ʱAl��
�������ʱAl��![]() ���ڼ�����
���ڼ�����![]() ��������Ӧ�Ļ�ѧ����ʽΪ��Al2O3+2OH-=2AlO2-+H2O��
��������Ӧ�Ļ�ѧ����ʽΪ��Al2O3+2OH-=2AlO2-+H2O��![]() ��ͬʱΪ�˱�֤����������ף�����NaOH���������ԣ���Һ�е�������Ϊ��AlO2-��OH-���ʴ�Ϊ��AlO2-��OH-��
��ͬʱΪ�˱�֤����������ף�����NaOH���������ԣ���Һ�е�������Ϊ��AlO2-��OH-���ʴ�Ϊ��AlO2-��OH-��
![]() ������������Ŀ�����Ʊ�
������������Ŀ�����Ʊ�![]() ���壬�������ʱ���õ���ӦΪ
���壬�������ʱ���õ���ӦΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
![]() �����γ�
�����γ�![]() �Ӷ��ﵽȥ��FeԪ�ص�Ŀ�ģ����ԣ������������������
�Ӷ��ﵽȥ��FeԪ�ص�Ŀ�ģ����ԣ������������������![]() ��Һ����
��Һ����![]() ����Ϊ
����Ϊ![]() �����ӷ���ʽΪ��H2O2+2Fe2++2H+=2H2O+2Fe3+�����ŷ�Ӧ�Ľ��У�������Fe3+���ܻ����������ֽ��ˮ�����������ݾ��������������������ݿ�֪����������Ksp=10-39��
�����ӷ���ʽΪ��H2O2+2Fe2++2H+=2H2O+2Fe3+�����ŷ�Ӧ�Ľ��У�������Fe3+���ܻ����������ֽ��ˮ�����������ݾ��������������������ݿ�֪����������Ksp=10-39��![]() ǡ�ó�����ȫ����
ǡ�ó�����ȫ����![]() ʱ����10-39=
ʱ����10-39=![]() ����Kw=c(H+)��C(OH-)�ã�c3(H+)=
����Kw=c(H+)��C(OH-)�ã�c3(H+)=![]() =10-8��c(H+)=
=10-8��c(H+)=![]() =
=![]() ��10-3������pH=-lg(
��10-3������pH=-lg(![]() ��10-3)=3-
��10-3)=3-![]() ��2.7,�ʴ�Ϊ��H2O2+2Fe2++2H+=2H2O+2Fe3+��Fe3+��H2O2�ֽ����O2��2.7 ��
��2.7,�ʴ�Ϊ��H2O2+2Fe2++2H+=2H2O+2Fe3+��Fe3+��H2O2�ֽ����O2��2.7 ��
![]() ��Һ�д���
��Һ�д���![]() ����������Ũ���Ͳ���A�õ�
����������Ũ���Ͳ���A�õ�![]() ���壬�����AΪ��ȴ�ᾧ���ʴ�Ϊ����ȴ�ᾧ��
���壬�����AΪ��ȴ�ᾧ���ʴ�Ϊ����ȴ�ᾧ��
(5)������FeCl3��AlCl3��ˮ�⣬Ϊ������FeCl3��AlCl3ˮ�⣬����FeCl3��AlCl3��Һʱ�������Ƚ�����FeCl3��AlCl3���ڽ�Ũ�����ᣬ��������ˮϡ�͵�����Ũ�ȣ�����ȷ��
��2FeCl3+Fe=3FeCl2��2Fe+3Cl2![]() 2FeCl3��4Fe(OH)2+2H2O+O2=4Fe(OH)3���ǻ��Ϸ�Ӧ������ȷ��
2FeCl3��4Fe(OH)2+2H2O+O2=4Fe(OH)3���ǻ��Ϸ�Ӧ������ȷ��
��Fe3+����Fe2+�ļ��飬������
��Al2(SO4)3+3H2O=2Al(OH)3+3H2SO4����������Al2(SO4)3ˮ�⣬�ʼ�������Al2(SO4)3��Һ��������ijɷ�Al2(SO4)3��������
�ʴ�Ϊ���٢���

����Ŀ���п�Ժһ�����³ɹ�ʵ���˼����Ч������ϩ�������ڴ����������⣬�������о����ɻ�ż����Ӧ������ϩ���䷴Ӧ���£�2CH4(g)C2H4(g)��2H2(g)����H��0
��ѧ�� | H��H | C��H | C��C | C��C |
E(kJ/mol) | a | b | c | d |
��1����֪��ػ�ѧ���ļ������ϱ��������Ʊ���ϩ��Ӧ�Ħ�H��____________ (�ú�a��b��c��d�Ĵ���ʽ��ʾ)��
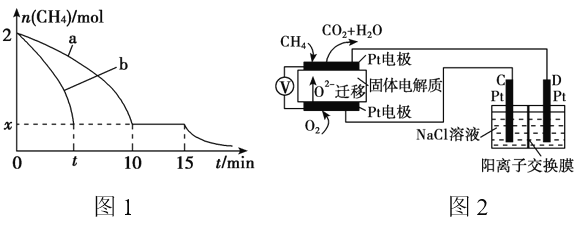
��2��T1�¶�ʱ����1 L�ĺ��ݷ�Ӧ���г���2mol CH4 ��������������Ӧ����Ӧ������ 0��15min CH4�����ʵ�����ʱ��仯��ͼ1�����10��15minʱH2��Ũ��Ϊ1.6mol/L��
��0��10min��CH4��ʾ�ķ�Ӧ����Ϊ__________mol/(L��min)��
����ͼ1������a������b�ֱ��ʾ���¶�T1ʱ��ʹ��������ͬ���������ͬ�Ĵ���ʱ���ﵽƽ�������n(CH4)�仯���ߣ����б�ʾ����������ϴ�������� ________ (�a���� ��b��)��
��15minʱ�����ı���練Ӧ����������n(CH4)����ͼ1����ʾ�仯����ı������������_____________________________________(�δ�һ������)��
��3��ʵ����v����k��c2(CH4)��v����k��c(C2H4)��c2(H2)����k����k��Ϊ���ʳ��������¶��йأ�T1�¶�ʱk����k���ı�ֵΪ______ (����ֵ)�������¶���T1���ߵ�T2����Ӧ��������ı���v��____v��(�>����������<��)���жϵ�������_________________ ��
��4��������Ա����˼���ȼ�ϵ�ز����ڵ�⡣��ͼ2��ʾ��������Dz����� Y2O3�� ZrO2�Ĺ��壬���ڸ����´���O2����
��C����PtΪ______ ��(����������� )��
�ڸõ�ع���ʱ������Ӧ����ʽΪ_____________________��
���øõ�ص�ⱥ��ʳ��ˮ��һ��ʱ����ռ�����������������Ϊ112mL����������������Һ��25��ʱpH��_______ (������ǰ��NaCl��Һ�������Ϊ500mL)��