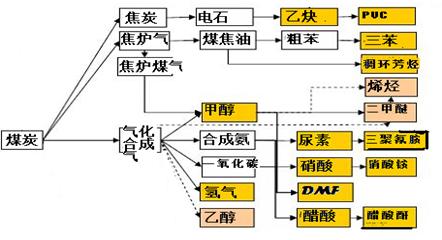
��Ŀ����
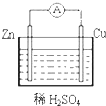
��9�֣��е��װ����ͼ4-37��ͼ��Bװ��ʢ1 L 2 mol��L-1 Na2SO4��Һ��Aװ����ʢ?1 L 2 mol��L-1 AgNO3��Һ��ͨ�����ʪKI������ֽ��C�˱���ɫ�����һ��ʱ����Իش�
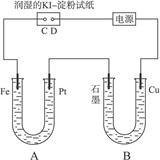
ͼ4-37
(1)A�з�����Ӧ�Ļ�ѧ����ʽΪ______________________________��
(2)��B�й۲쵽��������_________________________��
(3)�����£����ӵ�ʼ��ʱ��Ϊtʱ��A��Bװ���й��ռ�������0.168 L(��״��)����������������������Ӧ����������Һ����仯���Բ��ƣ�����tʱ��A��Һ��pHΪ_______��

ͼ4-37
(1)A�з�����Ӧ�Ļ�ѧ����ʽΪ______________________________��
(2)��B�й۲쵽��������_________________________��
(3)�����£����ӵ�ʼ��ʱ��Ϊtʱ��A��Bװ���й��ռ�������0.168 L(��״��)����������������������Ӧ����������Һ����仯���Բ��ƣ�����tʱ��A��Һ��pHΪ_______��
(1)4AgNO3+2H2O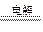 4Ag+O2��+4HNO3
4Ag+O2��+4HNO3
(2)ʯī�缫���������ݲ�����ͭ�缫��Χ��Һ����ɫ��һ��ʱ���U�ι��²�����ɫ�������� (3)2
 4Ag+O2��+4HNO3
4Ag+O2��+4HNO3(2)ʯī�缫���������ݲ�����ͭ�缫��Χ��Һ����ɫ��һ��ʱ���U�ι��²�����ɫ�������� (3)2
C�˱���˵�������˷�Ӧ2I--2e-====I2��ʹ���۱�������C��Ϊ������D��Ϊ�������ݴ����ȷ����Դ��������������A��B����������������B��ʯīΪ����������2H++2e-====H2��������OH-����CuΪ�������ʲ����ܽ⡣����A��FeΪ��������Ag������PtΪ������O2���������ڲ�����O2ΪH2�� ����O2�����ʵ���Ϊ
����O2�����ʵ���Ϊ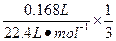 ="0.0025" mol��ͬʱ��ʹA��H+��Ϊ4��0.0025 mol="0.01" mol������A��c(H+)="0.01" mol��L-1��pH=2��
="0.0025" mol��ͬʱ��ʹA��H+��Ϊ4��0.0025 mol="0.01" mol������A��c(H+)="0.01" mol��L-1��pH=2��
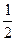 ����O2�����ʵ���Ϊ
����O2�����ʵ���Ϊ ="0.0025" mol��ͬʱ��ʹA��H+��Ϊ4��0.0025 mol="0.01" mol������A��c(H+)="0.01" mol��L-1��pH=2��
="0.0025" mol��ͬʱ��ʹA��H+��Ϊ4��0.0025 mol="0.01" mol������A��c(H+)="0.01" mol��L-1��pH=2��
��ϰ��ϵ�д�
�����Ŀ
 Fe(s)��CO2(g) ��H>0����֪��1100��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=0.263��
Fe(s)��CO2(g) ��H>0����֪��1100��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=0.263��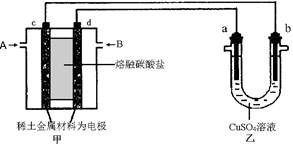
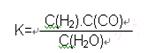
 2CO��g����K1
2CO��g����K1 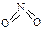 ��
��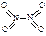 ����֪N��N������Ϊ167kJ��mol��1��NO2�е������ļ���Ϊ466kJ��mol��1��N2O4�е������ļ���Ϊ438.5kJ��mol��1����д��NO2ת��ΪN2O4���Ȼ�ѧ����ʽΪ ��
����֪N��N������Ϊ167kJ��mol��1��NO2�е������ļ���Ϊ466kJ��mol��1��N2O4�е������ļ���Ϊ438.5kJ��mol��1����д��NO2ת��ΪN2O4���Ȼ�ѧ����ʽΪ �� ��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ��
��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ��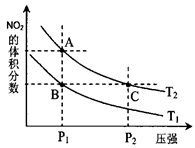
 2SO3��Ϊ���淴Ӧ����ش�
2SO3��Ϊ���淴Ӧ����ش�
 N2+2CO2��
N2+2CO2��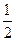 O2��g��=2CO2��g�� ��H=һ283 kJ��mol
O2��g��=2CO2��g�� ��H=һ283 kJ��mol ����
����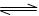 2NH3 (g) ��H<0�����з�����ȷ���� �� ��
2NH3 (g) ��H<0�����з�����ȷ���� �� ��