��Ŀ����
��16�֣���ͼ��ú������ҵ����һ���֣���������ѧ֪ʶ������������⣺
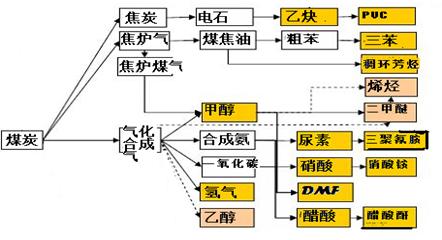
��1���ò�ҵ���кϳɰ��ķ�Ӧ�ڽϵ������ܷ��Է����У� ��
��2����֪�ò�ҵ����ij��Ӧ��ƽ�����ʽΪ��
������Ӧ�Ļ�ѧ��ӦΪ�� ��
��3����֪��һ���¶��£�����Ӧ��ƽ�ⳣ�����£�
C��s��+CO2��g�� 2CO��g����K1
2CO��g����K1
CO��g��+H2O��g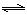 H2��g��+CO2��g����K2
H2��g��+CO2��g����K2
C��s��+H2O��g��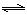 CO��g��+H2��g����K3
CO��g��+H2��g����K3
��K1��K2��K3֮��Ĺ�ϵ�ǣ� ��
��4��ú����ͨ��ͨ���о���ͬ�¶���ƽ�ⳣ���Խ������ʵ�����⡣��֪�������һ����̼��ˮ�������뷴Ӧ��ʱ���ᷢ�����·�Ӧ��CO(g)+H2O(g)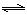 H2(g)+CO2(g)���÷�Ӧƽ�ⳣ�����¶ȵı仯���£�
H2(g)+CO2(g)���÷�Ӧƽ�ⳣ�����¶ȵı仯���£�
�÷�Ӧ������Ӧ������ ��Ӧ������ȡ����ȡ���������500��ʱ���У�����ʼʱCO��H2O����ʼŨ�Ⱦ�Ϊ0.020mol/L���ڸ������£�CO��ƽ��ת����Ϊ�� ��
��5����ͼ�п��������������������ᣬ�˹������漰���������NO��NO2��N2O4�ȡ���֪NO2��N2O4�Ľṹʽ�ֱ���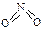 ��
��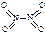 ����֪N��N������Ϊ167kJ��mol��1��NO2�е������ļ���Ϊ466kJ��mol��1��N2O4�е������ļ���Ϊ438.5kJ��mol��1����д��NO2ת��ΪN2O4���Ȼ�ѧ����ʽΪ ��
����֪N��N������Ϊ167kJ��mol��1��NO2�е������ļ���Ϊ466kJ��mol��1��N2O4�е������ļ���Ϊ438.5kJ��mol��1����д��NO2ת��ΪN2O4���Ȼ�ѧ����ʽΪ ��
�Է�ӦN2O4(g)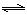 2NO2(g)�����¶�ΪT1
2NO2(g)�����¶�ΪT1 ��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ��
��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ��
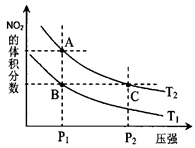
����˵����ȷ���ǡ���������
A��A��C�� ��ķ�Ӧ���ʣ�A��C
��ķ�Ӧ���ʣ�A��C
B��A��C�����������ɫ��A�Cdz
C��B��C����������ƽ����Է���������B��C
D����״̬B��״̬A�������ü��ȵķ���
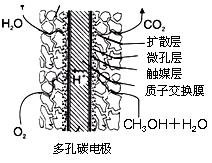
��6����������ҵ���м״�Ϊȼ���Ƴ�ȼ�ϵ�أ���д�����������ؽ����иõ�صĸ�����Ӧʽ ��

��1���ò�ҵ���кϳɰ��ķ�Ӧ�ڽϵ������ܷ��Է����У� ��
��2����֪�ò�ҵ����ij��Ӧ��ƽ�����ʽΪ��
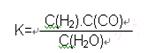
������Ӧ�Ļ�ѧ��ӦΪ�� ��
��3����֪��һ���¶��£�����Ӧ��ƽ�ⳣ�����£�
C��s��+CO2��g��
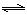 2CO��g����K1
2CO��g����K1 CO��g��+H2O��g
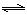 H2��g��+CO2��g����K2
H2��g��+CO2��g����K2 C��s��+H2O��g��
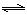 CO��g��+H2��g����K3
CO��g��+H2��g����K3��K1��K2��K3֮��Ĺ�ϵ�ǣ� ��
��4��ú����ͨ��ͨ���о���ͬ�¶���ƽ�ⳣ���Խ������ʵ�����⡣��֪�������һ����̼��ˮ�������뷴Ӧ��ʱ���ᷢ�����·�Ӧ��CO(g)+H2O(g)
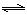 H2(g)+CO2(g)���÷�Ӧƽ�ⳣ�����¶ȵı仯���£�
H2(g)+CO2(g)���÷�Ӧƽ�ⳣ�����¶ȵı仯���£�| �¶�/�� | 400 | 500 | 800 |
| ƽ�ⳣ��K | 9.94 | 9 | 1 |
��5����ͼ�п��������������������ᣬ�˹������漰���������NO��NO2��N2O4�ȡ���֪NO2��N2O4�Ľṹʽ�ֱ���
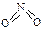 ��
��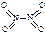 ����֪N��N������Ϊ167kJ��mol��1��NO2�е������ļ���Ϊ466kJ��mol��1��N2O4�е������ļ���Ϊ438.5kJ��mol��1����д��NO2ת��ΪN2O4���Ȼ�ѧ����ʽΪ ��
����֪N��N������Ϊ167kJ��mol��1��NO2�е������ļ���Ϊ466kJ��mol��1��N2O4�е������ļ���Ϊ438.5kJ��mol��1����д��NO2ת��ΪN2O4���Ȼ�ѧ����ʽΪ ���Է�ӦN2O4(g)
 2NO2(g)�����¶�ΪT1
2NO2(g)�����¶�ΪT1 ��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ��
��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ��
����˵����ȷ���ǡ���������
A��A��C��
 ��ķ�Ӧ���ʣ�A��C
��ķ�Ӧ���ʣ�A��C B��A��C�����������ɫ��A�Cdz
C��B��C����������ƽ����Է���������B��C
D����״̬B��״̬A�������ü��ȵķ���
��6����������ҵ���м״�Ϊȼ���Ƴ�ȼ�ϵ�أ���д�����������ؽ����иõ�صĸ�����Ӧʽ ��
��16�֣�
��1������
��2��C��s��+H2O��g�� CO��g��+H2��g��
CO��g��+H2��g��
��3��K3="K1��K2"
��4�����ȣ�75%
��5��2NO2(g) N2O4(g)����H����57 kJ��mol��1 �� D
N2O4(g)����H����57 kJ��mol��1 �� D
��6��CH3OH+8OH����6e��==CO32��+6H2O
��1������
��2��C��s��+H2O��g��
 CO��g��+H2��g��
CO��g��+H2��g����3��K3="K1��K2"
��4�����ȣ�75%
��5��2NO2(g)
 N2O4(g)����H����57 kJ��mol��1 �� D
N2O4(g)����H����57 kJ��mol��1 �� D ��6��CH3OH+8OH����6e��==CO32��+6H2O
��

��ϰ��ϵ�д�
 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
�����Ŀ
 Si3N4��s��+6CO��g��
Si3N4��s��+6CO��g�� C(g)��D(g)���仯ѧƽ�ⳣ��K���¶ȱ仯�Ĺ�ϵ���±���ʾ���������й��ж���ȷ����
C(g)��D(g)���仯ѧƽ�ⳣ��K���¶ȱ仯�Ĺ�ϵ���±���ʾ���������й��ж���ȷ����
 ���NOx������������Ͷ�����̼���������ɻ�����Ⱦ����ȼú���������������������̼�ȴ�������ʵ����ɫ���������ܼ��š��������õ�Ŀ�ġ�
���NOx������������Ͷ�����̼���������ɻ�����Ⱦ����ȼú���������������������̼�ȴ�������ʵ����ɫ���������ܼ��š��������õ�Ŀ�ġ� O��g����H2����1160 kJ��mol��1
O��g����H2����1160 kJ��mol��1 ��2����̼����CO2ת��Ϊ�״����Ȼ�ѧ����ʽΪ��
��2����̼����CO2ת��Ϊ�״����Ȼ�ѧ����ʽΪ�� CH3OH��g����H2O��g�� ��H3
CH3OH��g����H2O��g�� ��H3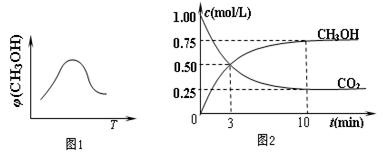
 ���г���1 mol CO2��3 mol H2������������Ӧ�����CO2��CH3OH��g����Ũ����ʱ��仯��ͼ2��ʾ������˵����ȷ���� ������ĸ���ţ���
���г���1 mol CO2��3 mol H2������������Ӧ�����CO2��CH3OH��g����Ũ����ʱ��仯��ͼ2��ʾ������˵����ȷ���� ������ĸ���ţ���