��Ŀ����
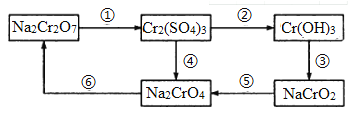
����Ŀ�����������(Na2S2O3��5H2O) ���������մ�������ˮ���������Ҵ����е�Ϊ100�棬���Ի�����������ֽ⡣ijʵ����ģ�ҵ���ȡ��������ƣ��䷴Ӧװ�ü������Լ���ͼ��

�ش��������⣺
��1��װ��A�����ڹ۲�SO2���������ʣ����е�Һ�����ѡ��_____________(�����)����װ�ö���ʹ������������A�е�����Ϊ_______________________________��
a.����ˮ b.����Na2SO3��Һ c.����NaHSO3��Һ d.����NaHCO3��Һ
��2��β����������ѡ������װ���е�________(�����)��

��3����Ӧ��������pHֵС��7��ή�Ͳ��ʣ��������ӷ���ʽ����ԭ��_________________________��
��4����д��װ��C�з�����Ӧ�Ļ�ѧ����ʽ��_________________________��
��5����Ӧ������ȡC�л������ȹ��ˣ�ȡ��Һ���������У� ______________�����ˣ�ϴ�ӣ������¸���ò�Ʒ��ϴ��ʱҪ�������ٲ�Ʒ����ʧ������������_________________________��
��6��Ϊ�����ƵõIJ�Ʒ�Ĵ��ȣ���ʵ��С���ȡ4g �IJ�Ʒ���Ƴ�250mL �����������Һ�����ü�ӵ������궨����Һ��Ũ�ȣ�����ƿ�м���20mL 0.0lmol��L-1KIO3��Һ�������������KI���ữ���������з�Ӧ��5I-+IO3-+6H+=3I2+3H2O���ټ��뼸�ε�����Һ�������������Na2S2O3��Һ�ζ���������Ӧ��I2+2S2O32-=2I-+ S4O62-��ʵ���������±���
ʵ����� | 1 | 2 | 3 |
Na2S2O3��Һ���(mL) | 19.98 | 20.02 | 21.2 |
���������һ��Na2S2O3��Һʱ����Һ___________���Ұ�����ڲ��仯����ﵽ�յ㡣����ò�Ʒ�Ĵ�����____________________________��
���𰸡� c ����©��Һ������ ab S2O32-+2H+= S��+H2O+SO2�� 2Na2S+Na2CO3+4SO2![]() 3 Na2S2O3+CO2 ˮԡ������������ȴ�ᾧ ���Ҵ���û�������еľ��壬��Ȼ���ɺ��ظ�2~3 �� ��ɫ��ȥ 93%
3 Na2S2O3+CO2 ˮԡ������������ȴ�ᾧ ���Ҵ���û�������еľ��壬��Ȼ���ɺ��ظ�2~3 �� ��ɫ��ȥ 93%
����������1��װ��A�����ڹ۲�SO2���������ʣ����ڶ�������������ˮ�������ڱ���NaHSO3��Һ�����ԣ����е�Һ�����ѡ��NaHSO3��Һ��ѡc����װ�ö���ʹ������������ɹ۲쵽A�е�����Ϊ������©��Һ��������
��2��β����������ѡ������װ���е�ab�����ǿ��Է�ֹ�����������������ܡ�
��3����Ӧ��������pHֵС��7��ή�Ͳ��ʣ���Ϊ���մ������Ի�����������ֽ��������ӷ���ʽΪ��S2O32-+2H+= S��+H2O+SO2����
��4��װ��C�з�����Ӧ�Ļ�ѧ����ʽ��2Na2S+Na2CO3+4SO2![]() 3 Na2S2O3+CO2��
3 Na2S2O3+CO2��
��5����Ӧ������ȡC�л������ȹ��ˣ�ȡ��Һ���������У� ˮԡ������������ȴ�ᾧ�����ˣ�ϴ�ӣ������¸���ò�Ʒ��ϴ��ʱҪ�������ٲ�Ʒ����ʧ��������֪�����մ�������ˮ���������Ҵ��������������������Ҵ���û�������еľ��壬��Ȼ���ɺ��ظ�2~3 �Ρ�
��6��Ϊ�����ƵõIJ�Ʒ�Ĵ��ȣ���ʵ��С���ȡ4g �IJ�Ʒ���Ƴ�250mL �����������Һ�����ü�ӵ������궨����Һ��Ũ�ȣ�����ƿ�м���20mL 0.0lmol��L-1KIO3��Һ�������������KI���ữ���������з�Ӧ��5I-+IO3-+6H+=3I2+3H2O���ټ��뼸�ε�����Һ�������������Na2S2O3��Һ�ζ���������Ӧ��I2+2S2O32-=2I-+ S4O62-��������ʵ�����ݿ�֪����3��ʵ���������ϴ�Ӧ��ȥ���ã�����������ε�ƽ��ֵΪ20.00mL���ɹ�ϵʽIO3- ~ 3I2 ~ 6S2O32-��֪��n(S2O32-)=6n(IO3-)=6![]() 20
20![]() 0.0lmol��L-1=1.2
0.0lmol��L-1=1.2![]() mol��
mol��
���������һ��Na2S2O3��Һʱ����Һ��ɫ��ȥ���Ұ�����ڲ��仯����ﵽ�յ㡣�ò�Ʒ�Ĵ�����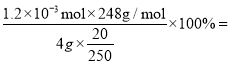 93%��
93%��