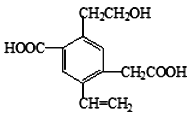
��Ŀ����
����Ŀ��298Kʱ����20mLcmol��L��1KOH��Һ�еμ�0.1mol��L��1HCOOH��Һ�������Һ��ˮ���������������Ũ����μӼ���(����)��Һ���(V)�Ĺ�ϵ��ͼ��ʾ������˵����ȷ����
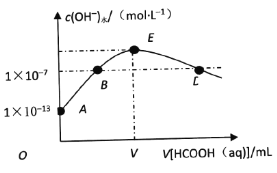
A. ����KOH��Һ��Ũ��c��0.01mol��L��1
B. B���Ӧ����Һ�У�c(K��)��c(HCOO��)
C. E���Ӧ����Һ�У�c(OH��)��c(H��)��c(HCOOH)
D. ��D���Ӧ�ļ�����Һ���ΪV1mL����HCOOH����ƽ�ⳣ��![]()
���𰸡�D
��������
�۲�ͼ���֪��E���ʾKOH��HCOOHǡ����ȫ��Ӧ����HCOOK����ʱ��Һ�ʼ��ԣ�B���Ӧ��Һ�е�������KOH��HCOOK����Һ�Լ��ԣ�D���Ӧ��Һ�е�������HCOOH��HCOOK�������ԣ��ݴ˷������
A������ͼ��ʼʱ��A���pH=13��˵��KOH��Һ��Ũ��c��0.1mol��L��1����A����
B��B���Ӧ��Һ�е�������KOH��HCOOK����Һ�Լ��ԣ�c(OH��)��c(H��)�����ݵ���غ��У�c(K��)��c(HCOO��)����B����
C��E��ΪKOH��HCOOHǡ����ȫ��Ӧ����HCOOK����ʱ��Һ�ʼ��Ը��������غ���c(OH��)=c(H��)+c(HCOOH)����c(H��)��c(HCOOH)��һ����ȣ���C����
D��D���Ӧ��Һ�е�������HCOOH��HCOOK�������ԣ�����c(K��)= c(HCOO��)=![]() =
= ![]() mol��L��1��c(HCOOH)=
mol��L��1��c(HCOOH)=![]() =
= ![]() mol��L��1����HCOOH����ƽ�ⳣ��
mol��L��1����HCOOH����ƽ�ⳣ��![]() =
=![]() =
= ![]() ����D��ȷ��
����D��ȷ��
��ѡD��

����Ŀ��I�������ڹ�ҵ�ϳ���Ӧ�ù㷺��
��1��ͨ�����з�Ӧ�����Ʊ��״�
��CO��g��+2H2��g��=CH3OH��g�� ��H=��90.8kJ��mol��1
��CO2��g��+H2��g��=CO��g��+H2O��g�� ��H=+41.3kJ��mol��1
��д����CO2��H2��ȡ�״����Ȼ�ѧ����ʽ��______________________��
��CH4�������������������Ⱦ����Ҫ��Ӧԭ��Ϊ
CH4��g��+2NO2��g��=CO2��g��+2H2O��g��+N2��g�� ��H=��868.7kJ��mol��1
��2����3.00L�ܱ�������ͨ��1 mol CH4��2 mol NO2����һ���¶��½���������Ӧ����Ӧʱ�䣨t����������������ѹǿ��p�������ݼ��±���
��Ӧʱ��t/min | 0 | 2 | 4 | 6 | 8 | 10 |
��ѹǿP/��100kPa | 4.80 | 5.44 | 5.76 | 5.92 | 6.00 | 6.00 |
�ɱ������ݼ��㣬0~4min��v��NO2��=___________�����¶��µ�ƽ�ⳣ��K=___________��
��3����һ����װ����ͨ��һ����CH4��NO2���������ͬʱ���ںͲ�ͬ�¶��£�NO2��ת��������ͼ��������������ȷ����___________��

A ���¶�ά����200�����ʱ�䣬NO2��ת���ʽ�����19%
B ��Ӧ����b���v���棩>e��ģ��棩
C ƽ�ⳣ����c��=d��
D b�㷴Ӧδ�ﵽƽ��
��4�����ð���������Ƹ��ܻ���ȼ�ϵ�أ��øõ�ص�⺬��NO3���ļ��Թ�ҵ��ˮ������������N2�������ĵ缫��ӦʽΪ______________________���ڱ�״���£��������ռ���13.44LN2ʱ������������NH3�����Ϊ___________��
��5�������£��ð�ˮ����CO2�ɵõ�NH4HCO3��Һ���� NH4HCO3��Һ��c��NH4+��___________c��HCO3����������>����<������=��������ӦNH4++HCO3��+H2O![]() NH3��H2O+H2CO3��ƽ�ⳣ��K=___________������֪������NH3��H2O�ĵ���ƽ�ⳣ��Kb=2��10��5��H2CO3�ĵ���ƽ�ⳣ��K1=4��10��7��K2=4��10��11��
NH3��H2O+H2CO3��ƽ�ⳣ��K=___________������֪������NH3��H2O�ĵ���ƽ�ⳣ��Kb=2��10��5��H2CO3�ĵ���ƽ�ⳣ��K1=4��10��7��K2=4��10��11��
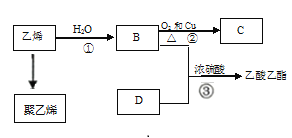
����Ŀ��CH3OH��һ����Ҫ�Ļ���ԭ�ϡ���ҵ�ϳ���CO��H2�Ļ������Ϊԭ���Ʊ��״����䷴Ӧ����ʽΪCO+2H2CH3OH��
(1)һ����������2 L���ܱ�������ͨ��һ������CO��H2ʹ�䷢��������Ӧ��n(CO)��ʱ��ı仯���±���ʾ:
ʱ��/min | 0 | 1 | 2 | 3 | 4 | 5 |
n(CO)/mol | 1.0 | 0.8 | 0.5 | 0.25 | 0.20 | 0.20 |
��ƽ����Ӧ��������ʱ���:___________min(�� ��0~1������1~2�� ����2~3����3~4������4~5��)��
�ڴӷ�Ӧ��ʼ��3 minĩ��������ƽ����Ӧ����v(H2)=______________mol��L-1��min-1
���ܹ��жϸ÷�Ӧ�ﵽƽ�����________(����ĸ) ��
a.v��(CO)=2v��(H2)
b.CO��H2��CH3OH�������ʵ�Ũ�����
c.CH3OH�ֽ�����ʺ�CH3OH���ɵ��������
d.��ͬʱ��������1molCO��ͬʱ����1molCH3OH
�ܹ�ҵ����CO��H2Ϊԭ���Ʊ��״��Ĺ����У����и����������(CH3OCH3)���ɣ�д���÷�Ӧ�Ļ�ѧ����ʽ:__________.
(2)�״�ȼ�ϵ����Ŀǰ������ɹ���ȼ�ϵ��֮һ������ȼ�ϵ���ɼ״���������KOH��Һ���ɣ�����ܷ�ӦΪ2CH3OH+3O2+4OH-=2![]() + 6H2O����װ��ͼ��ͼ��ʾ��
+ 6H2O����װ��ͼ��ͼ��ʾ��
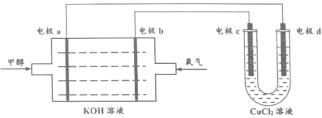
�ٵ缫aΪ��ص�_______(�� ������������������)
�ڵ缫b�ĵ缫��Ӧʽ:________��
�۵缫C�ĵ缫��Ӧʽ:________��
����Ŀ��ij��ѧ��ȤС��Ϊ��̽�����缫�ڵ���е����ã���Ʋ�����������һϵ��ʵ�飬ʵ������¼���£�
��� | �缫���� | �������Һ | ������ָ�� |
1 | Mg��Al | ϡ���� | ƫת |
2 | Al��Cu | ϡ���� | ƫת |
3 | Al��C(ʯī) | ϡ���� | ƫת |
4 | Mg��Al | ����������Һ | ƫת |
5 | Al��Zn | Ũ���� | ƫת |
���лش�������
A.ʵ��1��2���������ĵ缫(������)����ͬ
B.ʵ��2��3�����缫�ĵ缫��Ӧʽ��ΪAl��3e-��Al3+
C.ʵ��4�����缫�ĵ缫��ӦʽΪAl��3e-��4OH-��![]() +2H2O
+2H2O
D.ʵ��5����ʼ����ԭ��صĸ���
����Ŀ���±���Ԫ�����ڱ���һ����,�����Ԫ����-���ڱ��е�λ��,�ش��������⡣
�� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
2 | �� | �� | �� | �� | ||||
3 | �� | �� | �� | �� | �� | �� |
(1)����ԭ�ӽṹʾ��ͼ��___________��
(2)��ѧ��������õ�Ԫ����___________(��Ԫ�ط���)��
(3)������ԭ���У�ԭ�Ӱ뾶�ϴ����___________(��Ԫ�ط���)��
(4)�������뵼����ϵ���___________(������)��
(5)������������������Ӧ��ˮ���������Խ�ǿ����_________(�ѧʽ)��
(6)��ɫ��Ӧ�Ի�ɫ�����������Ľ���Ԫ����___________(��Ԫ�ط���)��
(7)Ԫ������������Ӧ��ˮ�����У������Ե���___________(�ѧʽ)��
(8)�ṹ��ʽΪ![]() ���л������ʽ��_____������̼Ԫ������Ԫ�ص�������m(C)��(H)=________��
���л������ʽ��_____������̼Ԫ������Ԫ�ص�������m(C)��(H)=________��