��Ŀ����
15�����ڵؿ��еĺ���Լռ 5%���ң�����ʯ������ܶ࣬��Ҫ���д�����ʯ����Ҫ�ɷ���Fe3O4����������ʯ����Ҫ�ɷ��� Fe2O3���ȣ���l��д����ҵ�ϸ�¯�����Ļ�ѧ����ʽ��Fe2O3+3CO$\frac{\underline{\;\;��\;\;}}{\;}$2Fe+3CO2��
��2�����ȵ����ܸ�ˮ������Ӧ��һ�ֲ�������������Ҫ�ɷ���ͬ����һ�ֲ����ǿ�ȼ�����壬���䷴Ӧ�Ļ�ѧ����ʽΪ3Fe+4H2O$\frac{\underline{\;����\;}}{\;}$Fe3O4+4H2��
��3���ڳ����£�����ˮ����Ӧ�����ڳ�ʪ�Ŀ����У������������⣨�������Ҫ�ɷ��� Fe203��������ʴ��ÿ����ʴ����ʧ�ĸֲ�ռ���������������ķ�֮һ��������Ļ�ѧԭ�����£����������ӷ���ʽ�ͻ�ѧ����ʽ����������
��ԭ��ط�Ӧ��������2Fe-4e-�T2Fe2+��������O2+4e-+2H2O=4OH-
������������γɣ�Fe2++2OH-�TFe��OH��2����4Fe��OH��2+O2+2H2O=4Fe��OH��3
����������ķֽ⣺2Fe��OH��3�TFe2O3+3H2O��
��4��Ϊ�˷�ֹ�����⣬��������Ʒ�������һ��ͭ������ͼװ�ã�����ֱ����Դa�������������������������������Ʒ���淢���ĵ缫��ӦʽΪCu2++2e-=Cu��

���� ��1����ҵ����һ����̼��ԭ������������
��2������ˮ������Ӧ����������������������
��3���ڳ�ʪ�Ļ����£���������������ʴ�����������õ������������������ӣ�������ʧȥ�������ɶ��������ӣ���������������ǿ�Ļ�ԭ�����ױ������е�����������������������
��4��������Ʒ�������һ��ͭ�������ͭ������������������������Ǻ���ͭ���ӵ��Σ����ݵ��صĹ���ԭ�����ش�
��� �⣺��1��һ����̼����������Ӧ�������������̼����ѧ����ʽ��Fe2O3+3CO$\frac{\underline{\;\;��\;\;}}{\;}$2Fe+3CO2��
�ʴ�Ϊ��Fe2O3+3CO$\frac{\underline{\;\;��\;\;}}{\;}$2Fe+3CO2��
��2������ˮ������Ӧ������������������������ѧ����ʽ��3Fe+4H2O$\frac{\underline{\;����\;}}{\;}$Fe3O4+4H2��
�ʴ�Ϊ��3Fe+4H2O$\frac{\underline{\;����\;}}{\;}$Fe3O4+4H2��
��3���ڳ�ʪ�Ļ����£���������������ʴ�����������õ������������������ӣ��缫��ӦʽΪ��O2+4e-+2H2O=4OH-��
������������������ˮ��Ӧ����������������ѧ����ʽ��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
O2+4e-+2H2O=4OH-��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
��4��������Ʒ�������һ��ͭ�������ͭ���������͵�Դ�����������������������Ϸ�����ԭ��Ӧ����Cu2++2e-=Cu��
�ʴ�Ϊ��������Cu2++2e-=Cu��
���� ���⿼���˻�ѧ����ʽ���缫��Ӧʽ����д����ȷԭ��ء����ع���ԭ���ǽ���ؼ�����Ŀ�ѶȲ���

 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д�| A�� | Ca��HCO3��2��Һ�����Ca��OH��2��Һ��Ӧ��Ca2++HCO3-+OH-�TCaCO3��+H2O | |
| B�� | �ö��Ե缫���MgCl2��Һ��2Cl-+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$H2��+Cl2��+2OH- | |
| C�� | ����ͨ��ˮ�У�C12+H2O�T2H++C1-+C1O- | |
| D�� | �����ᱵ��ϡ���ᷴӦ��BaSO3+2H+�TBa2++SO2��+H2O |
| A�� | ���黯ѧ���ʱȽ��ȶ������ܱ��κ����������� | |
| B�� | ��ϩ�ͱ������и�ԭ�Ӷ���ͬһƽ�� | |
| C�� | ��ϩ�ͱ�����ʹ��ˮ��ɫ��������ɫԭ����ͬ | |
| D�� | ʯ�͵��ѻ��������ѽ⣬ú�ĸ����ǻ�ѧ�仯 |
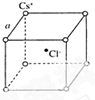
| A�� | �����������������������ȵ�ԭ�� | |
| B�� | M�������ΪK�������һ���ԭ�� | |
| C�� | �������2�����ӵ�ԭ�� | |
| D�� | M��������������ڲ������֮��$\frac{1}{5}$��ԭ�� |
 ������ѧ֪ʶ������ش��������⣺
������ѧ֪ʶ������ش��������⣺