��Ŀ����
14��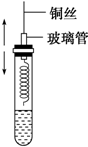 ijͬѧ��ѧϰ���Ҵ���֪ʶ���������ͼ��ʾ��ʵ�飮�������輰�۲쵽��������ͼ��
ijͬѧ��ѧϰ���Ҵ���֪ʶ���������ͼ��ʾ��ʵ�飮�������輰�۲쵽��������ͼ�������Թ������2mL�Ҵ���
�ڰ�һ���������״��ͭ˿���ھƾ��������м��ȱ�ڣ�
��������ͭ˿����ʢ���Ҵ����Թ��ͭ˿���±�Ϊ��ɫ�����������������Σ���ش��������⣺
��1����ʵ���Ŀ������֤�Ҵ��ڼ��Ⱥ�ͭ�����������£��ܱ�������������ȩ��
��2��д���ܵķ�Ӧ��ѧ����ʽ2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��
��3���ڴ˹����У�ͭ˿����������������
���� �������ͭ��O2��Ӧ����CuO�DZ��������������ͭ˿�ָ���ɫ��������CuO����ԭΪCu���Ҵ�������Ϊ��ȩ����Ȼ��ʵ���Ŀ������֤�Ҵ���������Ϊ��ȩ���Դ˽����⣮
��� �⣺��1����ʵ��ڢۿ�֪ͭ���ȱ�������������ͭ������ͭ���Ҵ���Ӧ������ȩ��ͭ����֪ʵ���Ŀ��Ϊ��֤�Ҵ��ڼ��Ⱥ�ͭ�����������£��ܱ�������������ȩ��
�ʴ�Ϊ����֤�Ҵ��ڼ��Ⱥ�ͭ�����������£��ܱ�������������ȩ��
��2����ͭ�������£��Ҵ�������������ȩ������ʽΪ2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O���ʴ�Ϊ��2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��
��3����Ӧǰ��ͭ���������䣬ͭ���������ã��ʴ�Ϊ����������
���� ���⿼���Ҵ�������̽����Ϊ��Ƶ���㣬������ѧ���ķ�����ʵ�������Ŀ��飬ע�����ʵ���ԭ���Լ��Ҵ������ʣ��ѶȲ���

��ϰ��ϵ�д�
 ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�
�����Ŀ
16�����Ƶ�˼ά�����ڻ�ѧѧϰ���о��г����������Ľ��ۣ�������Ƴ��Ľ�������Ҫ����ʵ���ļ������ȷ������ȷ������м������ƽ�������ȷ���ǣ�������
| A�� | ��CH4��NH4+��SO42-Ϊ��������ṹ�����Ʋ�PH4+��PO43-ҲΪ��������ṹ | |
| B�� | NaCl��CsCl��ѧʽ���ƣ���NaCl��CsCl�ľ���ṹҲ���� | |
| C�� | ������ʹ���Ը��������Һ��ɫ���ʱ���ͬϵ��Ҳ����ʹ���Ը��������Һ��ɫ | |
| D�� | CO2ͨ��Ca��ClO��2��Һ����CaCO3��HClO��SO2ͨ��Ca��ClO��2��ҺҲ����CaSO3��HClO |
5�������ֽ���G��L��M��R��������ʵ�飺
���ݱ�������ʵ�������ж������ֽ��������ǿ�����Ĵ�����ȷ���ǣ�������
| ʵ�� ���� | G | L | M | R |
| ����ˮ��Ӧ | ��Ӧ | ������Ӧ | δ�� | δ�� |
| ��2mol/L HCl ��Ӧ | �ܽⲢ�ų����� | �ܽⲢ�ų����� | ��Ӧ | ��Ӧ |
| ��Rn+��ˮ��Һ��Ӧ | �ܽⲢ�γɳ��� | δ�� | �ܽⲢ�γɳ��� | δ�� |
| A�� | L��G��R��M | B�� | G��L��M��R | C�� | L��G��M��R | D�� | L��R��G��M |
2�����������������ʵ����䣬�ȴ������Ӽ��ִ��ڹ��ۼ����ǣ�������
| A�� | MgCl2 | B�� | K2S | C�� | NaOH | D�� | SO3 |
9�����й����л�ѧ��û�б��ƻ����ǣ�������
| A�� | ˮ�������� | |
| B�� | ˮ���ȵ�1500�濪ʼ�ֽ� | |
| C�� | ���ڵ��Ȼ��� | |
| D�� | ú������˹����Ҫ�ɷ��Ǽ��飩����ը |
6������˵�����ʾ������ȷ�ģ�������
| A�� | ��֪C��s��+O2��g���TCO2��g����H1��C��s��+$\frac{1}{2}$O2��g���TCO ��g����H2�����H1����H2 | |
| B�� | ��ϡ��Һ�У�H+��aq��+OH-��aq���TH2O��l����H=-57.3kJ•mol-1��������0.5molH2SO4��Ũ�����뺬1mol NaOH����Һ��ϣ��ų����ȴ���57.3 kJ | |
| C�� | ��C��ʯī���TC�����ʯ������H=+1.90kJ•mol-1��֪�����ʯ��ʯī�ȶ� | |
| D�� | ��֪�����ı�ȼ����Ϊ-285.8 kJ•mol-1����Ӧ���Ȼ�ѧ����ʽΪ2H2��g��+O2��g��=2H2O��l����H=-285.8kJ•mol-1 |
3��������ʵ������йص��ǣ�������
| A�� | ˮ���ȵ��ܸ��¶ȶ����Էֽ� | |
| B�� | ����Һ��������������ˮ | |
| C�� | CH4��SiH4��GeH4��SnH4�۵�����Է���������������� | |
| D�� | HF��HCl��HBr��HI�����ȶ������μ��� |
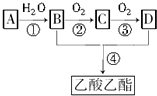
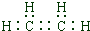 ��
�� CH3COOCH2CH3+H2O������ȡ����Ӧ��
CH3COOCH2CH3+H2O������ȡ����Ӧ��