��Ŀ����
17������ʵ�鷽�����ܴﵽʵ��Ŀ���ǣ�������| ʵ��Ŀ�� | ʵ�鷽�� | |
| A | �����������ƹ����Ƿ���� | ȡ�����������Թ��У���ˮ�ܽ⣬�μ����ᱵ��Һ������ɫ�������μ����ᣬ�۲�����Ƿ��ܽ� |
| B | �ⶨþ��������������������� | ȡmg������������NaOH��Һ����ַ�Ӧ���ˡ�ϴ�ӡ���ɣ�ʣ������ag������ |
| C | ����ij��ɫ��Һ��˵������NH4+ | ȡ������ɫ��Һ���Թ��У��μ�NaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�������ɵ����壬�۲���ֽ�Ƿ���� |
| D | ��ȥMgCl2��Һ�е�����FeCl3 | ����Һ�м�������Mg��OH��2��ĩ����ֽ��衢���á����� |
| A�� | A | B�� | B | C�� | C | D�� | D |
���� A������������������������¿�����������ӷ���������ԭ��Ӧ��
B���������������Ʒ�Ӧ����ƫ�����ƺ�������þ����Ӧ��
C�������Ӧ���ɰ�������ʹʪ��ĺ�ɫʯ����ֽ������
D��FeCl3��ˮ����������������
��� �⣺A������������������������¿�����������ӷ���������ԭ��Ӧ������������ӣ����ܹ۲쵽�����ܽ⣬��A����
B���������������Ʒ�Ӧ����ƫ�����ƺ�������þ����Ӧ��ʣ�����Ϊagþ������þ��������mg�����ɼ���þ������������Ȼ��������������������B��ȷ��
C����ij��Һ����NaOH���ȣ������ܹ�ʹʪ��ĺ�ɫʯ����ֽ��������ɫ���壬��������Ϊ�����������Һ��һ������NH4+����C��ȷ��
D��FeCl3��ˮ��������������������Mg��OH��2��ĩ��������Һ��pH���ٽ������ӵ�ˮ�⣬�Ҳ������µ����ʣ���D��ȷ��
��ѡA��
���� �������ڳ���ʵ�������ʵ�鿼�鷶�룬ע����������ʣ������ѶȲ���ע�����֪ʶ�Ļ��ۣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
7����ѧ������������أ������й�˵����ȷ���ǣ�������
| A�� | Li������Ľ�����Ҳ�ǻ�Լ�ǿ�Ľ������������ص��������� | |
| B�� | �����г��õľ������װ���Ǹ��ݽ������������ʶ���Ƶ� | |
| C�� | �������ĵ����Ա�ͭ������ǿ�����Գ�����������ߡ����� | |
| D�� | NH3���������������������������� |
8����1mol CH4��һ�����ʵ�����Cl2��Ͼ��ȣ�����ɢ������CH4��Cl2����ȡ����Ӧ����Ӧ��CH4��Cl2����ʣ�࣬�����ɵ����ʵ����������ȴ������μӷ�Ӧ��Cl2���ʵ���Ϊ��������
| A�� | 1.5mol | B�� | 2mol | C�� | 2.5mol | D�� | 4mol |
5������ɫ����������Һ�У��ܴ���������������ǣ�������
| A�� | Al3+��NH4+��Cl-��HCO3- | B�� | Na+��NO3-��SO42-��I- | ||
| C�� | Na+��Mg2+��Cl-��SO42- | D�� | Fe3+��K+��Cl-��NO3- |
12��ʵ���ҴӺ����Һ������H2O��CCl4��I2��I-�ȣ��л��յ⣬�����������£�

��1�����Һ�м���Na2SO3 ��Һ����������ԭ����Ӧ�����ӷ���ʽΪSO32-+I2+H2O=2I-+SO42-+2H+��
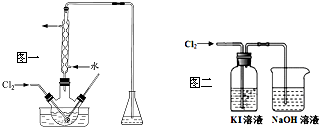
��2����������������������ƿ�н��У���ͼһ��������Һ���������pHԼΪ2������ͨ��Cl2��ʵ����ˮԡ������40�����ҵĽϵ��¶��½��е�ԭ����ʹ��������Һ���нϴ���ܽ�ȣ����ֹI2�������ֹI2��һ������������
��3��ij�о�С����ͼ��װ�ö�Cl2��KI��Һ�ķ�Ӧ����̽��������ͨ��Cl2һ��ʱ���KI��Һ��Ϊ��ɫ������ͨ��Cl2������Һ��ɫ��dz������Ϊ��ɫ���о�С���������ɫ��Һ�е�Ԫ�صĴ�����̬��������¼��裺
����һ��û��I2��̬���������û��I-��̬������������IO3-��̬��
�������ʵ��֤������һ�������Լ���ѡ����
������������������д������IO3-�����ӷ���ʽI2+5Cl2+6H2O=10Cl-+2IO3-+12H+��
��4�����о�С�黹�����˶Լӵ�����KIO3�����ⶨ������ʵ�飺
��ȷ��ȡ�ӵ���m g���ձ��У�������������ˮ������KI���ٵ���������ϡ���ᣬ��ַ�Ӧ�������û��Һ���250.00mL������Һ����ȡ25.00mL������Һ����ƿ�У��Ӽ��ε�����Һ����c mol•L-1 Na2S2O3��Һ�ζ����յ㣬�ظ�3�Σ����ƽ��ֵΪV mL��
��֪��IO3-+5I-+6H+=3H2O+3I2��I2+2S2O32-=2I-+S4O62-���ⶨʱ���жϴﵽ�ζ��յ������Ϊ��Һ����ɫ��Ϊ��ɫ���Ұ���Ӳ���ɫ���ɲⶨ���ݿ���ø���Ʒ�к�KIO3����������Ϊ$\frac{21.4CV}{6m}$%���ú�m��c��V�Ĵ���ʽ��ʾ��Mr��KIO3��=214 ����
���ڵζ�������ȷ���������£��ô��ֲⶨ������õĽ������ƫ�ߣ���ԭ�����ܿ�����Ӱ�죬�������ӷ���ʽ��ʾ������һӰ���ԭ��4I-+O2+4H+=2I2+2H2O��

��1�����Һ�м���Na2SO3 ��Һ����������ԭ����Ӧ�����ӷ���ʽΪSO32-+I2+H2O=2I-+SO42-+2H+��
��2����������������������ƿ�н��У���ͼһ��������Һ���������pHԼΪ2������ͨ��Cl2��ʵ����ˮԡ������40�����ҵĽϵ��¶��½��е�ԭ����ʹ��������Һ���нϴ���ܽ�ȣ����ֹI2�������ֹI2��һ������������
��3��ij�о�С����ͼ��װ�ö�Cl2��KI��Һ�ķ�Ӧ����̽��������ͨ��Cl2һ��ʱ���KI��Һ��Ϊ��ɫ������ͨ��Cl2������Һ��ɫ��dz������Ϊ��ɫ���о�С���������ɫ��Һ�е�Ԫ�صĴ�����̬��������¼��裺
����һ��û��I2��̬���������û��I-��̬������������IO3-��̬��
�������ʵ��֤������һ�������Լ���ѡ����
| ʵ����� | Ԥ������ | ���� |
| ����һ���� |
��4�����о�С�黹�����˶Լӵ�����KIO3�����ⶨ������ʵ�飺
��ȷ��ȡ�ӵ���m g���ձ��У�������������ˮ������KI���ٵ���������ϡ���ᣬ��ַ�Ӧ�������û��Һ���250.00mL������Һ����ȡ25.00mL������Һ����ƿ�У��Ӽ��ε�����Һ����c mol•L-1 Na2S2O3��Һ�ζ����յ㣬�ظ�3�Σ����ƽ��ֵΪV mL��
��֪��IO3-+5I-+6H+=3H2O+3I2��I2+2S2O32-=2I-+S4O62-���ⶨʱ���жϴﵽ�ζ��յ������Ϊ��Һ����ɫ��Ϊ��ɫ���Ұ���Ӳ���ɫ���ɲⶨ���ݿ���ø���Ʒ�к�KIO3����������Ϊ$\frac{21.4CV}{6m}$%���ú�m��c��V�Ĵ���ʽ��ʾ��Mr��KIO3��=214 ����
���ڵζ�������ȷ���������£��ô��ֲⶨ������õĽ������ƫ�ߣ���ԭ�����ܿ�����Ӱ�죬�������ӷ���ʽ��ʾ������һӰ���ԭ��4I-+O2+4H+=2I2+2H2O��

2������˵������ȷ���ǣ�������
| A�� | H2O�ڸ������ѷֽ⣬��H2S��300��ʱ�ͷֽ⣬˵��O�ǽ����Ա�Sǿ | |
| B�� | H2CO3�����Ա�HClO������ǿ������C�ķǽ����Ա�Clǿ | |
| C�� | NaOH�ļ��Ա�Mg��OH��2�ļ���ǿ������Na�Ľ����Ա�Mgǿ | |
| D�� | Fe3+�������Ա�Cu2+��������ǿ����Fe�Ľ����Ա�Cuǿ |
3������һ�����ǻ����л���A ��7g������һ�������·�Ӧ������10.2g������ij��������������1g ����ʣ�࣬��A�Ľṹ��ʽ�����ǣ�������
| A�� | CH3CH2OH | B�� | CH3CH2CH2OH | C�� | HOCH2CH2CHO | D�� |  |