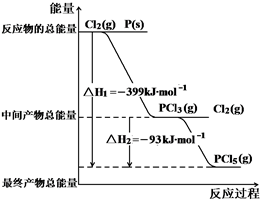题目内容
(1) 20世纪30年代,Eyring和Pzer在碰撞理论的基础上提出化学反应的过渡态理论:化学反应并不是通过简单的碰撞就能完成的,而是在反应物到生成物的过程中经过一个高能量的过渡态。如图是NO2和CO反应生成CO2和NO过程中的能量变化示意图, 说明这个反应是 (填“吸热”或“放热”)反应,NO2和CO的 总能量 (填“大于”、“小于”或“等于”)CO2和NO的总能量。
说明这个反应是 (填“吸热”或“放热”)反应,NO2和CO的 总能量 (填“大于”、“小于”或“等于”)CO2和NO的总能量。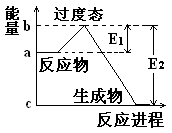
(2)在某体积为2L的密闭容器中充入1.5mol NO2和2mol CO,在一定条件下发生反应:NO2+CO CO2+NO,2 min时,测得容器中NO的物质的量为0.5 mol ,则:①此段时间内,用CO2表示的平均反应速率为 ②2 min时,容器内气体的总物质的量为_________.
CO2+NO,2 min时,测得容器中NO的物质的量为0.5 mol ,则:①此段时间内,用CO2表示的平均反应速率为 ②2 min时,容器内气体的总物质的量为_________.
(1) 放热 大于
(2)0.125mol/Lmin 3.5mol
解析试题分析:(1)反应物的总能量高于生成物的总能量,因此该反应时放热反应。所以反应物二氧化氮与一氧化碳的总能量大于产物二氧化碳与一氧化氮的总能量。(2)据题意可算出一氧化氮的速率为0.5除以2再除以2等于0.125 mol/Lmin,化学速率之比等于化学计量数之比,而一氧化碳与一氧化氮的化学计量数比为1;1所以,二氧化碳的速率为0.125mol/Lmin 。NO2+CO CO2+NO,2 min生成了0.5摩尔的一氧化氮,则也会生成0.5摩尔的二氧化碳。同时也会消耗0.5摩尔的一氧化碳和二氧化氮。所以剩余的一氧化碳为2减去0.5等于1.5,剩余的二氧化氮为1.5减去0.5等于1摩尔。所以容器内气体的总物质的量为1.5+1+0.5+0.5等于3.5摩尔
CO2+NO,2 min生成了0.5摩尔的一氧化氮,则也会生成0.5摩尔的二氧化碳。同时也会消耗0.5摩尔的一氧化碳和二氧化氮。所以剩余的一氧化碳为2减去0.5等于1.5,剩余的二氧化氮为1.5减去0.5等于1摩尔。所以容器内气体的总物质的量为1.5+1+0.5+0.5等于3.5摩尔
考点:考查化学反应与能量以及化学反应速率的相关知识

 53随堂测系列答案
53随堂测系列答案已知:2H2(g)+O2(g)=2H2O(l) ΔH=-571.6 kJ/mol,下列叙述正确的是( )
| A.2个氢分子和1个氧分子反应生成2个水分子,放出热量571.6 kJ |
| B.2 mol H2(g)和1 mol O2(g)反应生成2 mol H2O(l),吸收热量571.6 kJ |
| C.2 mol H2O(l)分解为2 mol H2(g)和1 mol O2(g),吸收热量571.6 kJ |
| D.2 mol H2(g)和1 mol O2(g)反应生成2 mol H2O(g),放出热量571.6 kJ |
(12分)(1)蕴藏在海底的“可燃冰”是高压下形成的外观像冰的甲烷水合物固体(分子式为CH4·9H2O),则356g“可燃冰”释放出的甲烷燃烧,生成液态水时能放出1780.6 kJ的热量,则甲烷燃烧的热化学方程式可表示为:_______________________________。
(2) 在100℃时,将0.100mol的N2O4气体充入1 L恒容抽空的密闭容器中,隔一定时间对该容器内物质的浓度进行分析得到下表数据: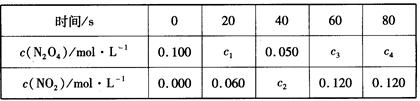
①从表中分析:该反应的平衡常数为___________;
②在上述条件下,60s内N2O4的平均反应速率为_____________;
③达平衡后下列条件的改变可使NO2浓度增大的是_________。
| A.增大容器的容积 | B.再充入一定量的N2O4 |
| C.再充入一定量的NO2 | D.再充入一定量的He |
②用等浓度的盐酸分别中和等体积浓度均为0.01mol/L的氨水和NaOH溶液,消耗盐酸的体积分别为V3、V4,则V3_____V4;
③用等浓度的盐酸分别和等体积浓度均为0.01mol/L的氨水和NaOH溶液反应,最后溶液均为中性,消耗盐酸的体积分别为V5、V6,则V5_____V6。
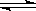 C达到平衡后,无论加压或降温,B的转化率都增大,则下列结论正确的是( )
C达到平衡后,无论加压或降温,B的转化率都增大,则下列结论正确的是( )

 CH3OH(g) ΔH1 ②CO2(g)+3H2(g)
CH3OH(g) ΔH1 ②CO2(g)+3H2(g)
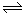 1/4CaS(s)+CO2(g) △H1=-47.3kJ/mol
1/4CaS(s)+CO2(g) △H1=-47.3kJ/mol