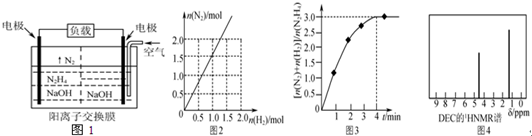
��Ŀ����
13����֪��S��g��+O2��g���TSO2��g������H1S��s��+O2��g���TSO2��g������H2
2H2S��g��+O2��g���T2S��s��+2H2O��l������H3
2H2S��g��+3O2��g���T2SO2��g��+2H2O��l������H4
SO2��g��+2H2S��g���T3S��s��+2H2O��l������H5
���й���������Ӧ�ʱ���жϲ���ȷ���ǣ�������
| A�� | ��H1����H2 | B�� | ��H3����H4 | C�� | ��H5=��H3-��H2 | D�� | 2��H5=3��H3-��H4 |
���� A����ӹ����Ϊ����Ĺ��������ȵĹ��̣�
B��������ȫ���ɶ�������ų�������Ҫ�Ȳ���ȫȼ���������ʷų��������ࣻ
C������ʽSO2��g��+2H2S��g���T3S��s��+2H2O��l�����Ը���2H2S��g��+O2��g���T2S��s��+2H2O��l����ȥS��s��+O2��g���TSO2��g���õ����ݸ�˹�������ش�
D������ʽSO2��g��+2H2S��g���T3S��s��+2H2O��l�����Ը���2H2S��g��+O2��g���T2S��s��+2H2O��l����2H2S��g��+3O2��g���T2SO2��g��+2H2O��l���õ����ݸ�˹�������ش�
��� �⣺A����ӹ����Ϊ����Ĺ��������ȵĹ��̣����ʵ�ȼ�շ��ȣ��ʱ��С���㣬���ԡ�H1����H2����A��ȷ��
B��������ȫ���ɶ�������ų�������Ҫ�Ȳ���ȫȼ���������ʷų��������࣬�����ȼ�ն��Ƿ��ȵģ��ʱ��Ǹ�ֵ�����ԡ�H3����H4����B����
C������ʽSO2��g��+2H2S��g���T3S��s��+2H2O��l�����Ը���2H2S��g��+O2��g���T2S��s��+2H2O��l����ȥS��s��+O2��g���TSO2��g���õ����ݸ�˹���ɡ�H5=��H3-��H2����C��ȷ��
D������ʽ2SO2��g��+4H2S��g���T6S��s��+4H2O��l�����Ը��ݷ�Ӧ3H2S��g��+3O2��g���T6S��s��+6H2O��l����ȥ2H2S��g��+3O2��g���T2SO2��g��+2H2O��l���õ����ݸ�˹���ɣ�2��H5=3��H3-��H4����D��ȷ��
��ѡB��
���� ���⿼�����ʱ䡢�ر���жϣ���ȷ��Ӧ��ЧӦ���ʱ�ֵ��С�Ĺ�ϵ�ǽ���ؼ���ע���˹���ɵ�Ӧ�ã���Ŀ�Ѷ��еȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�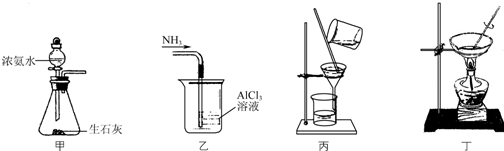
| A�� | ��װ�ü���ȡNH3 | |
| B�� | ��װ��������NH3��ȡAl��OH��3 | |
| C�� | ��װ�ñ��ڲ��Ͻ����·���Al��OH��3��NH4Cl��Һ | |
| D�� | ��װ�ö�����NH4Cl��Һ��������NH4Cl |
| A�� | 1��2��3������ | B�� | ���� | C�� | ����� | D�� | �����嶡�� |
| A�� | ������ʴ�ı����ǽ���ԭ��ʧȥ���Ӷ������� | |
| B�� | ����������ʴ��������Ӧ�ǣ�O2+4e-+2H2O=4OH- | |
| C�� | ����ȼ�ϵ���У������ڸ�������������Ӧ | |
| D�� | �����¸ֹ���ֱ����Դ���������������������ֹ� |
 ��
��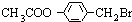 +3NaOH
+3NaOH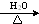 CH3COONa+
CH3COONa+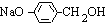 +NaBr+H2O��
+NaBr+H2O��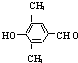 ��
�� ��
��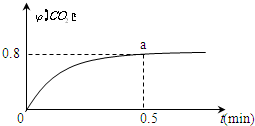 ������I2O5��s�������ڼ��CO����Ӧԭ��Ϊ��5CO��g��+I2O5��s��?5CO2��g��+I2��s����H��0��һ���¶��£���2L�����ܱ������м�������I2O5��s������ͨ��1molCO����Ӧ��CO2����������գ�CO2����ʱ��ı仯��ͼ��ʾ��
������I2O5��s�������ڼ��CO����Ӧԭ��Ϊ��5CO��g��+I2O5��s��?5CO2��g��+I2��s����H��0��һ���¶��£���2L�����ܱ������м�������I2O5��s������ͨ��1molCO����Ӧ��CO2����������գ�CO2����ʱ��ı仯��ͼ��ʾ��
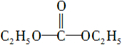 ��
��