��Ŀ����
1���������ʵ���Ũ�Ⱦ�Ϊ0.1mol/L����Һ��NH3•H2O��CH3COOH��KHSO4�������й�����Ũ�ȷ���һ������ȷ���ǣ�������| A�� | �������μ��������ڣ�$\frac{c��N{H}_{4}^{+}��}{c��O{H}^{-}��}$������ | |
| B�� | �١��۵������Ϻ���Һ�д��ڣ�NH4++H2O?NH3•H2O+H+ | |
| C�� | �١�������Ȼ�ϣ�c��CH3COO-��+c��OH-��=c��H+��+c��NH4+�� | |
| D�� | �١��۰������2��1��ϣ�c��NH4+����c��NH3•H2O����c��SO42-����c��OH-����c��H+�� |
���� A����NH3��H2O�еμ�CH3COOH������ǿ����ʴ���泥���Һ���Լ�����
B����NH3��H2O�е����ʵ�����KHSO4�����ɵ����ʵ�����K2SO4�ͣ�NH4��2SO4��笠�����ˮ�⣻
C���ݵ���غ������
D��NH3•H2O��KHSO4�����ʵ���֮��2��1��ϣ��õ���ͬŨ�ȵ�K2SO4�ͣ�NH4��2SO4��NH3•H2O�Ļ���һˮ�ϰ��ĵ���̶ȴ���笠���ˮ��̶ȣ�
��� �⣺A����NH3��H2O�еμ�CH3COOH��笠�����Ũ����������������Ũ�ȼ�С����$\frac{c��N{H}_{4}^{+}��}{c��O{H}^{-}��}$������A��ȷ��
B����NH3��H2O�е����ʵ�����KHSO4�����ɵ����ʵ�����K2SO4�ͣ�NH4��2SO4��笠�����ˮ�⣬笠�����ˮ������ӷ���ʽΪNH4++H2O?NH3•H2O+H+����B��ȷ��
C��NH3•H2O��CH3COOH�Ļ����Һ�д��ڵ���غ㣬ʼ�մ���c��CH3COO-��+c��OH-��=c��H+��+c��NH4+������C��ȷ��
D����ͬŨ�ȵ�K2SO4�ͣ�NH4��2SO4��NH3•H2O�Ļ����Һ�У�һˮ�ϰ��ĵ���̶ȴ���笠���ˮ��̶ȣ���������Ũ�ȵĴ�С˳��Ϊc��NH4+����c��SO42-����c��NH3•H2O����c��OH-����c��H+������D����
��ѡD��
���� ���⿼������Һ���ʱ������Ũ�ȵı仯�Լ���Һ�еĵ���غ㡢����Ũ�ȴ�С�Ƚϣ���Ŀ�ѶȲ���

 ����к͵ζ�����ѧ��ѧ����Ҫ�Ķ���ʵ��֮һ��
����к͵ζ�����ѧ��ѧ����Ҫ�Ķ���ʵ��֮һ����ij�о���ѧϰС��ȷ����������ʵ�飬��ȡ1.00g�����Ŀ�������Ʒ���250ml��Һ��ȡ��10.00ml������֪Ũ��Ϊ0.040mol•L-1��������еζ������ʲ������ᷴӦ����
����Ҫ��ش��������⣺
��1������250mL 0.040mol•L-1��������Һʱ��Ҫ�õ��IJ�����������Ͳ���ձ�������������ͷ�ιܺ�250mL����ƿ��
��2��Ϊ�ⶨ�ÿ�������Һ��ȷŨ�ȣ����εζ����������������£�
| ʵ����� | 1 | 2 | 3 | 4 |
| ����������Һ�������mL�� | 20.05 | 20.00 | 22.10 | 19.95 |
�ڸÿ�������Һ��Ũ��Ϊ0.080 mol•L-1��
��3��������������δ��������ϴ�ζ��ܣ��Բⶨ�����������Ӱ�죿��ƫ���ƫ����ƫС������Ӱ�족����
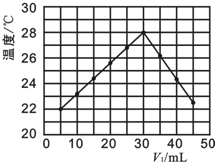
������һ��ʵ���У��о���С�齫V1 mL 1.0mol•L-1 HCl��Һ��V2 mL δ֪Ũ�ȵ�NaOH��Һ���Ȼ�Ϻ�������¼��Һ�¶ȣ�ʵ��������ͼ��ʾ��ʵ����ʼ�ձ���V1+V2=50mL����
��4��������������ȷ����B
A����ʵ��Ļ����¶�Ϊ22��
B����V1=40ʱ����Һ��c��Na+����c��Cl-��
C��NaOH��Һ��Ũ��Ϊ1.0mol•L-1
D�����������������䣬ֻ��HCl��ΪCH3COOH
����ʵ�飬Ҳ�õ���ͼ��ʵ������
��2C����̿��+O2��������$\frac{\underline{\;����\;}}{\;}$2CO��Fe2O3+3CO$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2
�����������У��Խ�̿��ʵ��ʹ����ҪԶԶ���ڰ��ջ�ѧ����ʽ������������Ҫԭ���ǣ�������
| A�� | CO���� | B�� | CO������ʯ�Ӵ������ | ||
| C�� | ������¯�ĸ߶Ȳ��� | D�� | CO��Fe2O3�ķ�Ӧ��һ���� |
| A�� | ����[KAl��SO4��2•12H2O]����ˮ���γɽ��壬��˿���������ˮ��ɱ������ | |
| B�� | С�մ�����������ȸ������ɼ�����������θ������һ��ҩ�� | |
| C�� | �����ʡ����ۡ���֬�ȶ�����������ˮ�Ⲣ�ṩ���� | |
| D�� | �����Ķ����������ִ���ѧ��������Ʒ�Ļ���ԭ�� |
| A�� | c��Na+��+c��H+��=c��OH-��+c��HS-��+c��S2-�� | B�� | c��Na+��=2c��HS-��+2c��S2-��+c��H2S�� | ||
| C�� | c��Na+����c��OH-����c��HS-����c��H+�� | D�� | c��H2S��+c��HS-��+c��H+��=c��OH-�� |
| A�� | �ð�ˮ���չ����Ķ�������NH3•H2O+SO2=NH4++HSO3- | |
| B�� | �ڳ���ʯ��ˮ��ͨ������������̼��OH-+CO2=HCO3- | |
| C�� | ���廯������ͨ������������2Br-+Cl2=2Cl-+Br2 | |
| D�� | �������������ն����������OH-+Cl2=2Cl-+HClO |
| A�� | ����ʱ��©���¶�Ҫ������Һ�ձ��ڱ� | |
| B�� | ����ʱ��Ӧʹ�¶ȼ�ˮ����������ƿ֧�ܿ� | |
| C�� | �����ᾧʱӦ����Һ���ɣ�Ȼ��ֹͣ���� | |
| D�� | ��Һʱ����Һ©���²�Һ����¿ڷų����ϲ�Һ����Ͽڵ��� |
| A�� | 0.10mol•L-1 | B�� | 0.15mol•L-1 | C�� | 0.225mol•L-1 | D�� | 0.30mol•L-1 |