��Ŀ����
9����ҵ�������ڸ�¯�н��еģ���¯��������Ҫ��Ӧ�ǣ���2C����̿��+O2��������$\frac{\underline{\;����\;}}{\;}$2CO��Fe2O3+3CO$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2
�����������У��Խ�̿��ʵ��ʹ����ҪԶԶ���ڰ��ջ�ѧ����ʽ������������Ҫԭ���ǣ�������
| A�� | CO���� | B�� | CO������ʯ�Ӵ������ | ||
| C�� | ������¯�ĸ߶Ȳ��� | D�� | CO��Fe2O3�ķ�Ӧ��һ���� |
���� ��ҵ������Fe2O3+3CO$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2Ϊ���淴Ӧ�����ӷ�Ӧ���Ͷ�ϣ����Դ�ʹ��ѧƽ��������У��ݴ��жϼ��ɣ�
��� �⣺��ҵ������Fe2O3+3CO$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2��Ϊ���淴Ӧ���Խ�̿��ʵ��ʹ����ҪԶԶ���ڰ��ջ�ѧ����ʽ�������裬�������Դٽ���Ӧ�����ƶ������CO��Ũ�ȣ��Ӷ�����ߵ�Fe2O3ת���ʣ���ѡD��
���� ������Ҫ���黯ѧƽ���ƶ�ԭ���ڹ�ҵ�����е����ã��ѶȲ�����ؼ���Ҫ��Ϥ��ҵ�����е��йط�Ӧ��

��ϰ��ϵ�д�
�����Ŀ
17����һ���¶��£�������X������Y��0.16mol����10.1���������������У�������ӦX��g��+Y��g��?2Z��g����H��0��һ��ʱ���ﵽƽ�⣬��Ӧ�����вⶨ���������������˵����ȷ���ǣ�������
| t/min | 2 | 4 | 7 | 9 |
| n��Y��mol | 0.12 | 0.11 | 0.10 | 0.10 |
| A�� | ��Ӧǰ2min��ƽ������x��Z��=2.0��10-3mol•L-1min-1 | |
| B�� | �����������䣬��ƽ����ϵ���ٳ���0.16mol����X����ԭƽ����ȣ��ﵽ��ƽ��ʱ������Y��ת��������X�������������Z������������� | |
| C�� | �����������䣬�����¶ȣ���Ӧ�ﵽ��ƽ��ǰv���棩��v������ | |
| D�� | ���������������䣬��ʼʱ����Һ�г���0.32mol����X��0.32mol����Y������ƽ��ʱ��n��Z����0.24mol |
4����֪��25��ʱ��Mg��OH��2��Ksp=5.61��10-12��MgF2��Ksp=7.42��10-11�������ж���ȷ���ǣ�������
| A�� | 25��ʱ������Mg��OH��2��Һ�뱥��MgF2��Һ��ȣ�ǰ�ߵ�c��Mg2+���� | |
| B�� | 25��ʱ����Mg��OH��2������Һ�м���������NH4Cl���壬c��Mg2+������ | |
| C�� | 25��ʱ��Mg��OH��2������20mL 0.01mol/L�İ�ˮ�е�Ksp����20mL0.01mol/L NH4Cl��Һ�е�KspС | |
| D�� | 25��ʱ����Mg��OH��2������Һ�м���NaF��Һ��Mg��OH��2������ת��ΪMgF2 |
14����ˮ��һ�ַḻ����Դ����ҵ�������ԴӺ�ˮ����ȡ�������õ����ʣ���Щ���ʹ㷺Ӧ��������������Ƽ������룬��ͼ1�Ǻ���CCl4��Һ�õ����ʵ����̣�
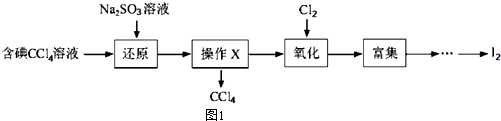
����������
��1������Һ�м����Թ�����Na2SO3��Һ�������ӷ���ʽΪSO32-+I2+H2O=2I-+SO42-+2H+�ò�����I2��ԭΪI��Ŀ���ǽ�I2��ԭΪI-��Ŀ����ʹ���Ȼ�̼�еĵⵥ��ת��Ϊ�����ӽ�����Һ
��2������X������Ϊ��Һ�����õ���Ҫ�����Ƿ�Һ©��
��3������ʱ��������ƿ�н�����I����ˮ��Һ���������pHԼΪ2��������ͨ��Cl2����40�����ҷ�Ӧ��ʵ��װ����ͼ2��ʾ����ʵ������ڽϵ��¶��½���ԭ���Ƿ�ֹ���������߱����������������ܽ����ƿ�е���ҺΪNaOH��Һ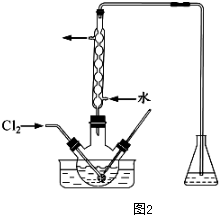
��4��Ũ��ˮ��ȡþ�Ĺ���������ͼ3��ʾ
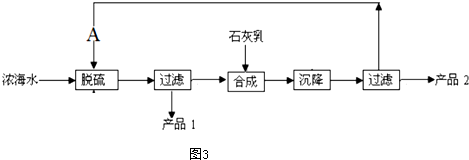
Ũ��ˮ����Ҫ�ɷ�����
�ù��չ����У��Ѽ����Ҫ��Ӧ�����ӷ���ʽΪCa2++SO42-=CaSO4����Ʒ2�Ļ�ѧʽΪMg��OH��2
1LŨ��ˮ���ɵõ���Ʒ2������Ϊ69.6g��

����������
��1������Һ�м����Թ�����Na2SO3��Һ�������ӷ���ʽΪSO32-+I2+H2O=2I-+SO42-+2H+�ò�����I2��ԭΪI��Ŀ���ǽ�I2��ԭΪI-��Ŀ����ʹ���Ȼ�̼�еĵⵥ��ת��Ϊ�����ӽ�����Һ
��2������X������Ϊ��Һ�����õ���Ҫ�����Ƿ�Һ©��
��3������ʱ��������ƿ�н�����I����ˮ��Һ���������pHԼΪ2��������ͨ��Cl2����40�����ҷ�Ӧ��ʵ��װ����ͼ2��ʾ����ʵ������ڽϵ��¶��½���ԭ���Ƿ�ֹ���������߱����������������ܽ����ƿ�е���ҺΪNaOH��Һ

��4��Ũ��ˮ��ȡþ�Ĺ���������ͼ3��ʾ

Ũ��ˮ����Ҫ�ɷ�����
| ���� | Na+ | Mg2+ | Cl- | SO${\;}_{4}^{2-}$ |
| Ũ��/��g��l-2�� | 63.7 | 28.8 | 144.6 | 46.4 |
1LŨ��ˮ���ɵõ���Ʒ2������Ϊ69.6g��
1���������ʵ���Ũ�Ⱦ�Ϊ0.1mol/L����Һ��NH3•H2O��CH3COOH��KHSO4�������й�����Ũ�ȷ���һ������ȷ���ǣ�������
| A�� | �������μ��������ڣ�$\frac{c��N{H}_{4}^{+}��}{c��O{H}^{-}��}$������ | |
| B�� | �١��۵������Ϻ���Һ�д��ڣ�NH4++H2O?NH3•H2O+H+ | |
| C�� | �١�������Ȼ�ϣ�c��CH3COO-��+c��OH-��=c��H+��+c��NH4+�� | |
| D�� | �١��۰������2��1��ϣ�c��NH4+����c��NH3•H2O����c��SO42-����c��OH-����c��H+�� |
18���������ļ�����ɵĻ������ͨ�����Һ�У�һ���ܲ����������ǣ�������
| �� | �� | �� | |
| A | NO2 | SO2 | BaCl2 |
| B | HCl | CO2 | ����ʯ��ˮ |
| C | CO2 | SO2 | ����ʯ��ˮ |
| D | CO2 | CO | CaCl2 |
| A�� | A | B�� | B | C�� | C | D�� | D |
19����2L���ܱ������н������·�Ӧ��CO��g��+H2O��g��?TCO2��g��+H2��g�������������ݣ�
����˵����ȷ���ǣ�������
| ʵ�� | �¶�/�� | ��ʼ��/mol | ƽ����/mol | |||
| CO | H2O | CO2 | H2 | CO2 | ||
| 1 | 650 | 2.0 | 1.0 | 0 | 0 | 0.8 |
| 2 | 800 | 2.0 | 2.0 | 0 | 0 | 1.0 |
| A�� | ����ӦΪ���ȷ�Ӧ | |
| B�� | ʵ��1�У�CO��ת����Ϊ80% | |
| C�� | 650��ʱ����ѧƽ�ⳣ��K=$\frac{8}{3}$ | |
| D�� | ʵ��1�ټ���1.0 mol H2O�����´ﵽƽ��ʱ��n��CO2��Ϊ1.6 mol |